Abstract
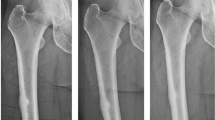
Recent reports of long-term bisphosphonate-treated patients developing cortical fractures have raised concerns that such fractures may relate to excessive suppression of bone turnover after prolonged use of these drugs. To evaluate the bone histology of patients presenting with cortical fractures after bisphosphonate therapy, we conducted a retrospective analysis of patients treated at Washington University Bone Health Program presenting with a history of low-energy cortical fractures (femoral shaft, pelvis, rib, metatarsal, and ankle), who had received bisphosphonates for at least two consecutive years and had undergone bone biopsy. Fifteen of 54 patients who underwent bone biopsy between November 2004 and March 2007 met the criteria. Of these, 10 patients had findings of suppressed trabecular bone remodeling, as demonstrated by lack of double tetracycline labels. There were no significant differences in bone density, clinical features, and biochemical features between those with suppressed turnover and the other five subjects with normal remodeling. However, the low-turnover group had received bisphosphonates (primarily alendronate) for a significantly longer duration (6.5 ± 0.6 vs. 3.9 ± 0.8 years, P = 0.02). Thus, about two-thirds of patients presenting with cortical fractures while on long-term treatment with bisphosphonates had suppressed turnover. Since the prevalence of such histological findings in nonfracture patients remains unknown, the impact of suppressed bone turnover on the development of cortical fractures cannot be determined. Considering the widespread use of bisphosphonates, it appears that the overall risk of cortical fractures is low. However, there may be a subset of as yet unidentified patients who could be predisposed to this complication.


Similar content being viewed by others
References
Black DM, Cummings SR, Karpf DB, Cauley JA, Thompson DE, Nevitt MC, Bauer DC, Genant HK, Haskell WL, Marcus R, Ott SM, Torner JC, Quandt SA, Reiss TF, Ensrud KE (1996) Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet 348:1535–1541
Orwoll E, Ettinger M, Weiss S, Miller P, Kendler D, Graham J, Adami S, Weber K, Lorenc R, Pietschmann P, Vandormael K, Lombardi A (2000) Alendronate for the treatment of osteoporosis in men. N Engl J Med 343:604–610
Liberman UA, Weiss SR, Broll J, Minne HW, Quan H, Bell NH, Rodriguez-Portales J, Downs RW Jr, Dequeker J, Favus M (1995) Effect of oral alendronate on bone mineral density and the incidence of fractures in postmenopausal osteoporosis. The Alendronate Phase III Osteoporosis Treatment Study Group. N Engl J Med 333:1437–1443
Cummings SR, Black DM, Thompson DE, Applegate WB, Barrett-Connor E, Musliner TA, Palermo L, Prineas R, Rubin SM, Scott JC, Vogt T, Wallace R, Yates AJ, LaCroix AZ (1998) Effect of alendronate on risk of fracture in women with low bone density but without vertebral fractures: results from the Fracture Intervention Trial. JAMA 280:2077–2082
Rosen CJ, Hochberg MC, Bonnick SL, McClung M, Miller P, Broy S, Kagan R, Chen E, Petruschke RA, Thompson DE, de Papp AE (2005) Treatment with once-weekly alendronate 70 mg compared with once-weekly risedronate 35 mg in women with postmenopausal osteoporosis: a randomized double-blind study. J Bone Miner Res 20:141–151
Odvina CV, Zerwekh JE, Rao DS, Maalouf N, Gottschalk FA, Pak CY (2005) Severely suppressed bone turnover: a potential complication of alendronate therapy. J Clin Endocrinol Metab 90:1294–1301
Armamento-Villareal R, Napoli N, Panwar V, Novack D (2006) Suppressed bone turnover during alendronate therapy for high-turnover osteoporosis. N Engl J Med 355:2048–2050
Lenart BA, Lorich DG, Lane JM (2008) Atypical fractures of the femoral diaphysis in postmenopausal women taking alendronate. N Engl J Med 358:1304–1306
Schneider JP (2006) Should bisphosphonates be continued indefinitely? An unusual fracture in a healthy woman on long-term alendronate. Geriatrics 61:31–33
Goh SK, Yang KY, Koh JS, Wong MK, Chua SY, Chua DT, Howe TS (2007) Subtrochanteric insufficiency fractures in patients on alendronate therapy: a caution. J Bone Joint Surg Br 89:349–353
Kwek EB, Goh SK, Koh JS, Png MA, Howe TS (2008) An emerging pattern of subtrochanteric stress fractures: a long-term complication of alendronate therapy? Injury 39:224–231
Parfitt AM, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR (1987) Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res 2:595–610
Armamento-Villareal RC, Napoli N, Klug T, Civitelli R (2004) The oxidative metabolism of estrogen modulates response to ERT/HRT in postmenopausal women. Bone 35:682–688
Cummings SR, Black DM, Nevitt MC, Browner W, Cauley J, Ensrud K, Genant HK, Palermo L, Scott J, Vogt TM (1993) Bone density at various sites for prediction of hip fractures. The Study of Osteoporotic Fractures Research Group. Lancet 341:72–75
Melton LJ III, Atkinson EJ, O’Fallon WM, Wahner HW, Riggs BL (1993) Long-term fracture prediction by bone mineral assessed at different skeletal sites. J Bone Miner Res 8:1227–1233
(1998) Osteoporosis: review of the evidence for prevention, diagnosis and treatment and cost-effectiveness analysis. Executive summary. Osteoporos Int 8(suppl 4):S3–S6
Mazziotti G, Angeli A, Bilezikian JP, Canalis E, Giustina A (2006) Glucocorticoid-induced osteoporosis: an update. Trends Endocrinol Metab 17:144–149
Lange U, Teichmann J, Muller-Ladner U, Strunk J (2005) Increase in bone mineral density of patients with rheumatoid arthritis treated with anti-TNF-alpha antibody: a prospective open-label pilot study. Rheumatology (Oxford) 44:1546–1548
Allali F, Breban M, Porcher R, Maillefert JF, Dougados M, Roux C (2003) Increase in bone mineral density of patients with spondyloarthropathy treated with anti-tumour necrosis factor alpha. Ann Rheum Dis 62:347–349
Delmas PD (2000) Markers of bone turnover for monitoring treatment of osteoporosis with antiresorptive drugs. Osteoporos Int 11(suppl 6):S66–S76
Van Staa TP, Laan RF, Barton IP, Cohen S, Reid DM, Cooper C (2003) Bone density threshold and other predictors of vertebral fracture in patients receiving oral glucocorticoid therapy. Arthritis Rheum 48:3224–3229
Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J (2002) Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA 288:321–333
Bone HG, Hosking D, Devogelaer JP, Tucci JR, Emkey RD, Tonino RP, Rodriguez-Portales JA, Downs RW, Gupta J, Santora AC, Liberman UA (2004) Ten years’ experience with alendronate for osteoporosis in postmenopausal women. N Engl J Med 350:1189–1199
Black DM, Schwartz AV, Ensrud KE, Cauley JA, Levis S, Quandt SA, Satterfield S, Wallace RB, Bauer DC, Palermo L, Wehren LE, Lombardi A, Santora AC, Cummings SR (2006) Effects of continuing or stopping alendronate after 5 years of treatment: the Fracture Intervention Trial Long-term Extension (FLEX): a randomized trial. JAMA 296:2927–2938
Mashiba T, Hirano T, Turner CH, Forwood MR, Johnston CC, Burr DB (2000) Suppressed bone turnover by bisphosphonates increases microdamage accumulation and reduces some biomechanical properties in dog rib. J Bone Miner Res 15:613–620
Sobelman OS, Gibeling JC, Stover SM, Hazelwood SJ, Yeh OC, Shelton DR, Martin RB (2004) Do microcracks decrease or increase fatigue resistance in cortical bone? J Biomech 37:1295–1303
Zioupos P (2001) Accumulation of in vivo fatigue microdamage and its relation to biomechanical properties in ageing human cortical bone. J Microsc 201:270–278
Nancollas GH, Tang R, Phipps RJ, Henneman Z, Gulde S, Wu W, Mangood A, Russell RG, Ebetino FH (2006) Novel insights into actions of bisphosphonates on bone: differences in interactions with hydroxyapatite. Bone 38:617–627
Orriss IR, Key ML, Colston KW, Arnett TR (2009) Inhibition of osteoblast function in vitro by aminobisphosphonates. J Cell Biochem 106:109–118
Chavassieux PM, Arlot ME, Roux JP, Portero N, Daifotis A, Yates AJ, Hamdy NA, Malice MP, Freedholm D, Meunier PJ (2000) Effects of alendronate on bone quality and remodeling in glucocorticoid-induced osteoporosis: a histomorphometric analysis of transiliac biopsies. J Bone Miner Res 15:754–762
Bone HG, Greenspan SL, McKeever C, Bell N, Davidson M, Downs RW, Emkey R, Meunier PJ, Miller SS, Mulloy AL, Recker RR, Weiss SR, Heyden N, Musliner T, Suryawanshi S, Yates AJ, Lombardi A (2000) Alendronate and estrogen effects in postmenopausal women with low bone mineral density. Alendronate/Estrogen Study Group. J Clin Endocrinol Metab 85:720–726
Acknowledgments
This work was supported in part by National Institutes of Health grant K12 HD01459 (Building Interdisciplinary Research Careers in Women’s Health, to R. A.-V.). These results were presented at the 29th annual meeting of the American Society for Bone and Mineral Research, Honolulu, HI, September 16–19, 2007.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Armamento-Villareal, R., Napoli, N., Diemer, K. et al. Bone Turnover in Bone Biopsies of Patients with Low-Energy Cortical Fractures Receiving Bisphosphonates: A Case Series. Calcif Tissue Int 85, 37–44 (2009). https://doi.org/10.1007/s00223-009-9263-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-009-9263-5