-
PDF
- Split View
-
Views
-
Cite
Cite
Sotiris C. Stamou, Robert C. Hagberg, Kamal R. Khabbaz, Mark R. Stiegel, Mark K. Reames, Eric Skipper, Marcy Nussbaum, Kevin W. Lobdell, Is advanced age a contraindication for emergent repair of acute type A aortic dissection?, Interactive CardioVascular and Thoracic Surgery, Volume 10, Issue 4, April 2010, Pages 539–544, https://doi.org/10.1510/icvts.2009.222984
Close - Share Icon Share
Abstract
With the general increase in human lifespan, cardiac surgeons are faced with treating an increasing number of elderly patients. The aim of our study was to investigate whether advanced age poses an increased risk for major morbidity and mortality with repair of acute type A aortic dissection. Between 2000 and 2008, 119 patients underwent emergency operation for acute type A aortic dissection at two institutions; 90 were younger than 70 years of age and 29 patients were 70 years or older. Major morbidity, operative and 5-year actuarial survival were compared between groups. The operative mortality rates were comparable between the two groups (18.9% in patients <70 years vs. 24.1% for patients ≥70 years, P=0.6). There was no difference in the rates of reoperation for bleeding (<70 years 31.7% vs. 14.3% for ≥70 years, P=0.09), stroke (18.9% for those <70 years vs. 20.7% for those ≥70 years, P=0.79), acute renal failure (22.2% for those <70 years vs. 17.2% for those ≥70 years, P=0.79) or prolonged ventilation (34.4% for those <70 years vs. 24.1% for those ≥70 years, P=0.36) between the two groups. Actuarial 5-year survival rates were 77% for patients <70 years vs. 59% for patients ≥70 years (P=0.07). The mortality for patients who presented with hemodynamic instability was markedly higher (10 out of 14 patients, 71.4%) compared with the mortality of those who presented with stable hemodynamics (21 out of 88 patients, 23.9%, P<0.001), regardless of age group. No significant differences in operative mortality, major morbidity and actuarial 5-year survival were observed between patients ≥70 years and younger patients although there was a trend toward a lower actuarial 5-year survival in older patients. Surgery for type A acute aortic dissection in patients 70 years or older can be performed with acceptable outcomes. Hemodynamic instability portends a poor prognosis, regardless of age.
1. Introduction
Cardiac surgeons are faced with the challenge of caring for an increasing number of elderly patients as a result of progressing aging of the Western population. Surgery for acute type A dissection poses an increased operative risk in the elderly population [1–7]. An ethical dilemma arises for the surgeon as to whether it is appropriate to deny offering surgery to elderly patients with acute type A aortic dissection solely on the basis of advanced age. Published studies have been inconsistent in their findings as to whether advanced age per se is a contraindication for repair of acute type A dissection [1–7].
Our study sought to investigate whether patients 70 years or older have a higher morbidity or mortality than those <70 years when undergoing repair of acute type A dissection.
2. Patients and methods
2.1. Patients
The databases of the Divisions of Cardiothoracic Surgery at the Beth Israel Deaconess Medical Center and the Sanger Heart and Vascular Institute were queried and identified 119 patients who underwent repair of acute type A dissection at either institution between January 2000 and July 2008. Computed tomography angiogram or transesophageal echocardiography were used to make the diagnosis which was confirmed at the time of operation. The operations were performed by the same group of cardiac surgeons in each institution for the study period. Baseline demographics, procedural data, and perioperative outcomes were recorded and entered prospectively in each institution's pre-specified database by dedicated data-coordinating personnel.
Long-term survival data were obtained from the national death index at the Center for Disease Control and Prevention website (http://ssdi.rootsweb.ancestry.com/cgi-bin/ssdi.cgi) and follow-up was 100% complete.
2.2. Study design and conduct
This is a retrospective cohort study of prospectively collected data from consecutive patients who underwent repair of acute type A dissection at the Beth Israel Deaconess and the Sanger Heart and Vascular Institute. Study approval was sought and obtained from the Institutional Review Board at each site. Patient confidentiality was maintained at all times, consistent with the Health Insurance Portability and Accountability Act of 1996 (HIPAA) regulations.
2.3. Definitions
The Society of Thoracic Surgeons' national cardiac surgery database definitions were used for this study. Acute type A dissection was defined as any dissection that involved the ascending aorta with presentation within two weeks of the onset of symptoms. Previous cerebrovascular accident was defined as history of central neurologic deficit persisting for more than 24 h. Chronic renal insufficiency was defined as a serum creatinine value ≥2.0 mg/dl. Diabetes was defined as a history of diabetes mellitus, regardless of duration of disease or need for oral agents or insulin. Recent myocardial infarction was defined as myocardial infarction occurring within seven days. Depressed ejection fraction was defined as ejection fraction <40%. Hemodynamic instability was defined as hypotension (systolic blood pressure <80 mmHg) or the presence of cardiac tamponade, shock, acute congestive heart failure, myocardial ischemia and/or infarction. Prolonged ventilatory support was defined as pulmonary insufficiency requiring ventilatory support >24 h, postoperatively. Postoperative stroke was defined as any new major (type I) neurologic deficit presenting in-hospital and persisting >72 h [8]. Acute renal failure was defined as one or both of the following: (1) an increase in the serum creatinine to >2.0 mg/dl and/or a >2-fold increase in the most recent preoperative creatinine level or (2) a new requirement for dialysis, postoperatively. Prolonged hospital length of stay was defined as hospital stay for more than 14 days. Operative mortality includes both (1) all deaths occurring during the hospitalization in which the operation was performed (even if death occurred after 30 days from the operation), and (2) those deaths occurring after discharge from the hospital, but within 30 days of the procedure.
2.4. Operative technique
A median sternotomy was performed and patients were placed on total cardiopulmonary bypass with venous cannulation of the right atrium and femoral or right axillary artery cannulation. Myocardial protection was obtained with cold blood cardioplegia solution given retrograde via the coronary sinus and/or antegrade directly down the ostia of the coronary arteries. A left ventricular vent was placed via the right superior pulmonary vein. Hypothermic circulatory arrest was used in most cases (63%) and the arch was inspected for tears in those patients. The goals of surgery were to resect the intimal tear, replace the ascending aorta, resuspend the aortic valve (or replace if repair was not feasible) and restore the anatomy of the aortic root. The proximal and distal suture lines were reinforced with Teflon (polytetrafluoroethylene) strips and in some cases biologic glue (BioGlue® surgical adhesive, Cryolife, Kennesaw, GA, USA) was used to reapproximate the dissected layers. If the aortic root was deemed to be involved beyond repair, then a root replacement was performed using a composite valve graft with coronary button reimplantation.
2.5. Data analysis
2.5.1. Univariate analysis
Univariate comparisons of preoperative, operative, and postoperative variables were performed between patients 70 years or older (n=29) and those younger than 70 years (n=90). Continuous variables were summarized using medians and ranges, while frequencies and percentages were reported for categorical variables. Continuous variables were tested using the Kruskal–Wallis test for association, while categorical variables were assessed by the χ2 or Fisher's exact test, depending upon the distribution of the data. The Cochran–Armitage test for trend was used for ordinal variables as appropriate.
2.5.2. Survival analysis
Kaplan–Meier unadjusted survival estimates were calculated for patients 70 years and older and then compared to those younger than 70 years using a log-rank test. All analyses were conducted using SAS statistical software version 9.2 (SAS Institute, Cary, NC, USA).
3. Results
3.1. Preoperative characteristics
Univariate comparisons between groups are presented in Table 1 . Patients ≥70 years were more likely to be females and have associated coronary artery disease compared to younger patients with type A aortic dissection. Younger patients were more likely to present with moderate or severe aortic insufficiency.
Preoperative patient characteristics
| Age <70 | Age ≥70 | P-value | |
| years | years | ||
| n=90 | n=29 | ||
| Age (median, range) | 56 (19–70) | 77 (70–87) | <0.001 |
| Female gender | 23% | 52% | 0.006 |
| Diabetes | 10% | 10% | >0.99 |
| Congestive heart failure | 12% | 10% | >0.99 |
| Hypertension | 77% | 83% | 0.61 |
| Chronic renal insufficiency | 10% | 7% | >0.99 |
| Chronic obstructive lung disease | |||
| None | 93% | 93% | 0.87 |
| Mild | 6% | 7% | |
| Moderate | 1 | 0% | |
| History of myocardial infarction | 10% | 17% | 0.33 |
| History of cerebrovascular disease | 9% | 7% | >0.99 |
| Unstable angina | 33% | 41% | 0.50 |
| Arrhythmia | 10% | 24% | 0.065 |
| Preoperative aortic insufficiency | 14% | 3.5% | 0.18 |
| (Moderate/Severe) | |||
| Hemodynamic instability | 16% | 8% | 0.51 |
| Number of diseased vessels | |||
| One | 3% | 25% | 0.038 |
| Two | 2% | 4% | |
| Three | 2% | 4% | |
| None | 93% | 67% |
| Age <70 | Age ≥70 | P-value | |
| years | years | ||
| n=90 | n=29 | ||
| Age (median, range) | 56 (19–70) | 77 (70–87) | <0.001 |
| Female gender | 23% | 52% | 0.006 |
| Diabetes | 10% | 10% | >0.99 |
| Congestive heart failure | 12% | 10% | >0.99 |
| Hypertension | 77% | 83% | 0.61 |
| Chronic renal insufficiency | 10% | 7% | >0.99 |
| Chronic obstructive lung disease | |||
| None | 93% | 93% | 0.87 |
| Mild | 6% | 7% | |
| Moderate | 1 | 0% | |
| History of myocardial infarction | 10% | 17% | 0.33 |
| History of cerebrovascular disease | 9% | 7% | >0.99 |
| Unstable angina | 33% | 41% | 0.50 |
| Arrhythmia | 10% | 24% | 0.065 |
| Preoperative aortic insufficiency | 14% | 3.5% | 0.18 |
| (Moderate/Severe) | |||
| Hemodynamic instability | 16% | 8% | 0.51 |
| Number of diseased vessels | |||
| One | 3% | 25% | 0.038 |
| Two | 2% | 4% | |
| Three | 2% | 4% | |
| None | 93% | 67% |
Preoperative patient characteristics
| Age <70 | Age ≥70 | P-value | |
| years | years | ||
| n=90 | n=29 | ||
| Age (median, range) | 56 (19–70) | 77 (70–87) | <0.001 |
| Female gender | 23% | 52% | 0.006 |
| Diabetes | 10% | 10% | >0.99 |
| Congestive heart failure | 12% | 10% | >0.99 |
| Hypertension | 77% | 83% | 0.61 |
| Chronic renal insufficiency | 10% | 7% | >0.99 |
| Chronic obstructive lung disease | |||
| None | 93% | 93% | 0.87 |
| Mild | 6% | 7% | |
| Moderate | 1 | 0% | |
| History of myocardial infarction | 10% | 17% | 0.33 |
| History of cerebrovascular disease | 9% | 7% | >0.99 |
| Unstable angina | 33% | 41% | 0.50 |
| Arrhythmia | 10% | 24% | 0.065 |
| Preoperative aortic insufficiency | 14% | 3.5% | 0.18 |
| (Moderate/Severe) | |||
| Hemodynamic instability | 16% | 8% | 0.51 |
| Number of diseased vessels | |||
| One | 3% | 25% | 0.038 |
| Two | 2% | 4% | |
| Three | 2% | 4% | |
| None | 93% | 67% |
| Age <70 | Age ≥70 | P-value | |
| years | years | ||
| n=90 | n=29 | ||
| Age (median, range) | 56 (19–70) | 77 (70–87) | <0.001 |
| Female gender | 23% | 52% | 0.006 |
| Diabetes | 10% | 10% | >0.99 |
| Congestive heart failure | 12% | 10% | >0.99 |
| Hypertension | 77% | 83% | 0.61 |
| Chronic renal insufficiency | 10% | 7% | >0.99 |
| Chronic obstructive lung disease | |||
| None | 93% | 93% | 0.87 |
| Mild | 6% | 7% | |
| Moderate | 1 | 0% | |
| History of myocardial infarction | 10% | 17% | 0.33 |
| History of cerebrovascular disease | 9% | 7% | >0.99 |
| Unstable angina | 33% | 41% | 0.50 |
| Arrhythmia | 10% | 24% | 0.065 |
| Preoperative aortic insufficiency | 14% | 3.5% | 0.18 |
| (Moderate/Severe) | |||
| Hemodynamic instability | 16% | 8% | 0.51 |
| Number of diseased vessels | |||
| One | 3% | 25% | 0.038 |
| Two | 2% | 4% | |
| Three | 2% | 4% | |
| None | 93% | 67% |
3.2. Operative characteristics
Operative patient characteristics are presented in Table 2 . Patients ≥70 years were more likely to have shorter circulatory arrest times and more likely to receive smaller aortic valve prosthesis, if replacement was performed, compared to younger patients. Patients ≥70 years were also more likely to have adjunctive coronary artery bypass grafting along with the aortic repair compared to younger patients. However, this difference was not found to be statistically significant. No differences were found between the two groups in terms of cross-clamp time, pump time or method of cannulation.
Operative patient characteristics
| Age <70 years | Age ≥70 years | P-value | |
| n=90 | n=29 | ||
| Coronary artery bypass grafting | 12% | 28% | 0.077 |
| Intraoperative blood products | 82% | 83% | >0.99 |
| Postoperative blood products | 77% | 90% | 0.18 |
| Cross-clamp time (median/range) | 91 (29–249) | 99 (46–215) | 0.77 |
| Perfusion time (median/range) | 151 (52–336) | 148 (94–302) | 0.61 |
| Circulatory arrest time (median/range) | 26 (3–90) | 13 (5–30) | 0.026 |
| Hypothermic circulatory arrest | 60% | 72% | 0.27 |
| Aortic implant type | |||
| Bioprosthesis | 11% | 14% | 0.41 |
| Mechanical | 12% | 3% | |
| Aortic implant size | |||
| 21 | 5% | 60% | 0.014 |
| 23 | 14% | 20% | |
| 25 | 33% | 0% | |
| 27 | 29% | 20% | |
| 29 | 14% | 0% | |
| 32 | 5% | 0% | |
| Aortic implant size (median/range) | 25 (21–32) | 21 (21–27) | 0.022 |
| Aortic valve procedure | |||
| Resuspension | 63% | 46% | 0.12 |
| Replacement | 12% | 15% | |
| Composite valve root conduit | 14% | 8% | |
| None | 11% | 31% | |
| Distal anastomotic technique | |||
| Open distal | 61% | 70% | 0.37 |
| Distal with cross-clamp | 39% | 30% | 0.51 |
| Hemi-arch technique | 46% | 65% | 0.087 |
| Total arch replacement | 11% | 3% | 0.29 |
| Arterial cannulation | |||
| Axillary artery | 12% | 8% | 0.60 |
| Femoral artery | 80% | 86% | |
| Both femoral and axillary arteries | 7% | 3% | |
| Aorta | 1% | 3% | |
| Retrograde cerebral perfusion | 5% | 0% | 0.57 |
| Antegrade cerebral perfusion | 21% | 14% | 0.59 |
| Use of biologic glue | 59% | 65% | 0.52 |
| Age <70 years | Age ≥70 years | P-value | |
| n=90 | n=29 | ||
| Coronary artery bypass grafting | 12% | 28% | 0.077 |
| Intraoperative blood products | 82% | 83% | >0.99 |
| Postoperative blood products | 77% | 90% | 0.18 |
| Cross-clamp time (median/range) | 91 (29–249) | 99 (46–215) | 0.77 |
| Perfusion time (median/range) | 151 (52–336) | 148 (94–302) | 0.61 |
| Circulatory arrest time (median/range) | 26 (3–90) | 13 (5–30) | 0.026 |
| Hypothermic circulatory arrest | 60% | 72% | 0.27 |
| Aortic implant type | |||
| Bioprosthesis | 11% | 14% | 0.41 |
| Mechanical | 12% | 3% | |
| Aortic implant size | |||
| 21 | 5% | 60% | 0.014 |
| 23 | 14% | 20% | |
| 25 | 33% | 0% | |
| 27 | 29% | 20% | |
| 29 | 14% | 0% | |
| 32 | 5% | 0% | |
| Aortic implant size (median/range) | 25 (21–32) | 21 (21–27) | 0.022 |
| Aortic valve procedure | |||
| Resuspension | 63% | 46% | 0.12 |
| Replacement | 12% | 15% | |
| Composite valve root conduit | 14% | 8% | |
| None | 11% | 31% | |
| Distal anastomotic technique | |||
| Open distal | 61% | 70% | 0.37 |
| Distal with cross-clamp | 39% | 30% | 0.51 |
| Hemi-arch technique | 46% | 65% | 0.087 |
| Total arch replacement | 11% | 3% | 0.29 |
| Arterial cannulation | |||
| Axillary artery | 12% | 8% | 0.60 |
| Femoral artery | 80% | 86% | |
| Both femoral and axillary arteries | 7% | 3% | |
| Aorta | 1% | 3% | |
| Retrograde cerebral perfusion | 5% | 0% | 0.57 |
| Antegrade cerebral perfusion | 21% | 14% | 0.59 |
| Use of biologic glue | 59% | 65% | 0.52 |
Operative patient characteristics
| Age <70 years | Age ≥70 years | P-value | |
| n=90 | n=29 | ||
| Coronary artery bypass grafting | 12% | 28% | 0.077 |
| Intraoperative blood products | 82% | 83% | >0.99 |
| Postoperative blood products | 77% | 90% | 0.18 |
| Cross-clamp time (median/range) | 91 (29–249) | 99 (46–215) | 0.77 |
| Perfusion time (median/range) | 151 (52–336) | 148 (94–302) | 0.61 |
| Circulatory arrest time (median/range) | 26 (3–90) | 13 (5–30) | 0.026 |
| Hypothermic circulatory arrest | 60% | 72% | 0.27 |
| Aortic implant type | |||
| Bioprosthesis | 11% | 14% | 0.41 |
| Mechanical | 12% | 3% | |
| Aortic implant size | |||
| 21 | 5% | 60% | 0.014 |
| 23 | 14% | 20% | |
| 25 | 33% | 0% | |
| 27 | 29% | 20% | |
| 29 | 14% | 0% | |
| 32 | 5% | 0% | |
| Aortic implant size (median/range) | 25 (21–32) | 21 (21–27) | 0.022 |
| Aortic valve procedure | |||
| Resuspension | 63% | 46% | 0.12 |
| Replacement | 12% | 15% | |
| Composite valve root conduit | 14% | 8% | |
| None | 11% | 31% | |
| Distal anastomotic technique | |||
| Open distal | 61% | 70% | 0.37 |
| Distal with cross-clamp | 39% | 30% | 0.51 |
| Hemi-arch technique | 46% | 65% | 0.087 |
| Total arch replacement | 11% | 3% | 0.29 |
| Arterial cannulation | |||
| Axillary artery | 12% | 8% | 0.60 |
| Femoral artery | 80% | 86% | |
| Both femoral and axillary arteries | 7% | 3% | |
| Aorta | 1% | 3% | |
| Retrograde cerebral perfusion | 5% | 0% | 0.57 |
| Antegrade cerebral perfusion | 21% | 14% | 0.59 |
| Use of biologic glue | 59% | 65% | 0.52 |
| Age <70 years | Age ≥70 years | P-value | |
| n=90 | n=29 | ||
| Coronary artery bypass grafting | 12% | 28% | 0.077 |
| Intraoperative blood products | 82% | 83% | >0.99 |
| Postoperative blood products | 77% | 90% | 0.18 |
| Cross-clamp time (median/range) | 91 (29–249) | 99 (46–215) | 0.77 |
| Perfusion time (median/range) | 151 (52–336) | 148 (94–302) | 0.61 |
| Circulatory arrest time (median/range) | 26 (3–90) | 13 (5–30) | 0.026 |
| Hypothermic circulatory arrest | 60% | 72% | 0.27 |
| Aortic implant type | |||
| Bioprosthesis | 11% | 14% | 0.41 |
| Mechanical | 12% | 3% | |
| Aortic implant size | |||
| 21 | 5% | 60% | 0.014 |
| 23 | 14% | 20% | |
| 25 | 33% | 0% | |
| 27 | 29% | 20% | |
| 29 | 14% | 0% | |
| 32 | 5% | 0% | |
| Aortic implant size (median/range) | 25 (21–32) | 21 (21–27) | 0.022 |
| Aortic valve procedure | |||
| Resuspension | 63% | 46% | 0.12 |
| Replacement | 12% | 15% | |
| Composite valve root conduit | 14% | 8% | |
| None | 11% | 31% | |
| Distal anastomotic technique | |||
| Open distal | 61% | 70% | 0.37 |
| Distal with cross-clamp | 39% | 30% | 0.51 |
| Hemi-arch technique | 46% | 65% | 0.087 |
| Total arch replacement | 11% | 3% | 0.29 |
| Arterial cannulation | |||
| Axillary artery | 12% | 8% | 0.60 |
| Femoral artery | 80% | 86% | |
| Both femoral and axillary arteries | 7% | 3% | |
| Aorta | 1% | 3% | |
| Retrograde cerebral perfusion | 5% | 0% | 0.57 |
| Antegrade cerebral perfusion | 21% | 14% | 0.59 |
| Use of biologic glue | 59% | 65% | 0.52 |
3.3. Postoperative characteristics
Postoperative characteristics are shown in Table 3 . No differences were documented in operative mortality and major morbidity between the two groups. Limb ischemia occurred in only two patients younger than 70 years (2.2%) and in no patients over 70 years (P>0.99). Seven patients <70 years vs. 4 patients >70 years developed cardiac arrest after surgery. The causes of cardiac arrest for the patients <70 years were acute myocardial infarction (n=1), ventricular tachyarrhythmia (n=2), stroke (n=3), respiratory failure (n=1) and for patients >70 years the causes of cardiac arrest were acute myocardial infarction (n=1), ventricular tachyarrhythmia (n=2) and respiratory failure (n=1). Of the seven patients <70 years who had postoperative cardiac arrest, six died. Of the four patients >70 years who had postoperative cardiac arrest, all four died. Hemodynamic instability was not found to be significantly different between the two groups but was a major risk factor for early mortality. The mortality for patients who presented with hemodynamic instability was markedly higher (10 out of 14 patients, 71.4%) compared with the mortality of those who presented with stable hemodynamics (21 out of 88 patients, 23.9%, P<0.001).
Postoperative patient characteristics
| Age <70 | Age ≥70 | P-value | |
| years | years | ||
| n=90 | n=29 | ||
| Reoperation for bleeding | 32% | 14% | 0.09 |
| Perioperative myocardial infarction | 2% | 3% | 0.57 |
| Mediastinitis | 2% | 0% | >0.99 |
| Septicemia | 12% | 7% | 0.73 |
| Acute renal failure | 22% | 17% | 0.79 |
| Hemodialysis | 8% | 14% | 0.46 |
| Prolonged ventilation (>24 h) | 34% | 24% | 0.36 |
| Cardiac arrest | 8% | 14% | 0.46 |
| Pericardial tamponade | 7% | 0% | 0.33 |
| Atrial fibrillation | 28% | 31% | 0.81 |
| Stroke | 19% | 21% | 0.79 |
| Prolonged length of stay (>14 days) | 48% | 41% | 0.67 |
| Length of stay (median/range) | 14 (0–70) | 14 (1–63) | 0.94 |
| Limb ischemia | 2% | 0% | >0.99 |
| Operative mortality | 19% | 24% | 0.60 |
| Cause of operative mortality | |||
| Cardiac | 47% | 72% | 0.098 |
| Infection | 0% | 14% | |
| Pulmonary | 6% | 0 | |
| Neurological | 41% | 0 | |
| Respiratory | 0% | 14% | |
| Other | 6% | 0 |
| Age <70 | Age ≥70 | P-value | |
| years | years | ||
| n=90 | n=29 | ||
| Reoperation for bleeding | 32% | 14% | 0.09 |
| Perioperative myocardial infarction | 2% | 3% | 0.57 |
| Mediastinitis | 2% | 0% | >0.99 |
| Septicemia | 12% | 7% | 0.73 |
| Acute renal failure | 22% | 17% | 0.79 |
| Hemodialysis | 8% | 14% | 0.46 |
| Prolonged ventilation (>24 h) | 34% | 24% | 0.36 |
| Cardiac arrest | 8% | 14% | 0.46 |
| Pericardial tamponade | 7% | 0% | 0.33 |
| Atrial fibrillation | 28% | 31% | 0.81 |
| Stroke | 19% | 21% | 0.79 |
| Prolonged length of stay (>14 days) | 48% | 41% | 0.67 |
| Length of stay (median/range) | 14 (0–70) | 14 (1–63) | 0.94 |
| Limb ischemia | 2% | 0% | >0.99 |
| Operative mortality | 19% | 24% | 0.60 |
| Cause of operative mortality | |||
| Cardiac | 47% | 72% | 0.098 |
| Infection | 0% | 14% | |
| Pulmonary | 6% | 0 | |
| Neurological | 41% | 0 | |
| Respiratory | 0% | 14% | |
| Other | 6% | 0 |
Postoperative patient characteristics
| Age <70 | Age ≥70 | P-value | |
| years | years | ||
| n=90 | n=29 | ||
| Reoperation for bleeding | 32% | 14% | 0.09 |
| Perioperative myocardial infarction | 2% | 3% | 0.57 |
| Mediastinitis | 2% | 0% | >0.99 |
| Septicemia | 12% | 7% | 0.73 |
| Acute renal failure | 22% | 17% | 0.79 |
| Hemodialysis | 8% | 14% | 0.46 |
| Prolonged ventilation (>24 h) | 34% | 24% | 0.36 |
| Cardiac arrest | 8% | 14% | 0.46 |
| Pericardial tamponade | 7% | 0% | 0.33 |
| Atrial fibrillation | 28% | 31% | 0.81 |
| Stroke | 19% | 21% | 0.79 |
| Prolonged length of stay (>14 days) | 48% | 41% | 0.67 |
| Length of stay (median/range) | 14 (0–70) | 14 (1–63) | 0.94 |
| Limb ischemia | 2% | 0% | >0.99 |
| Operative mortality | 19% | 24% | 0.60 |
| Cause of operative mortality | |||
| Cardiac | 47% | 72% | 0.098 |
| Infection | 0% | 14% | |
| Pulmonary | 6% | 0 | |
| Neurological | 41% | 0 | |
| Respiratory | 0% | 14% | |
| Other | 6% | 0 |
| Age <70 | Age ≥70 | P-value | |
| years | years | ||
| n=90 | n=29 | ||
| Reoperation for bleeding | 32% | 14% | 0.09 |
| Perioperative myocardial infarction | 2% | 3% | 0.57 |
| Mediastinitis | 2% | 0% | >0.99 |
| Septicemia | 12% | 7% | 0.73 |
| Acute renal failure | 22% | 17% | 0.79 |
| Hemodialysis | 8% | 14% | 0.46 |
| Prolonged ventilation (>24 h) | 34% | 24% | 0.36 |
| Cardiac arrest | 8% | 14% | 0.46 |
| Pericardial tamponade | 7% | 0% | 0.33 |
| Atrial fibrillation | 28% | 31% | 0.81 |
| Stroke | 19% | 21% | 0.79 |
| Prolonged length of stay (>14 days) | 48% | 41% | 0.67 |
| Length of stay (median/range) | 14 (0–70) | 14 (1–63) | 0.94 |
| Limb ischemia | 2% | 0% | >0.99 |
| Operative mortality | 19% | 24% | 0.60 |
| Cause of operative mortality | |||
| Cardiac | 47% | 72% | 0.098 |
| Infection | 0% | 14% | |
| Pulmonary | 6% | 0 | |
| Neurological | 41% | 0 | |
| Respiratory | 0% | 14% | |
| Other | 6% | 0 |
3.4. Survival analysis
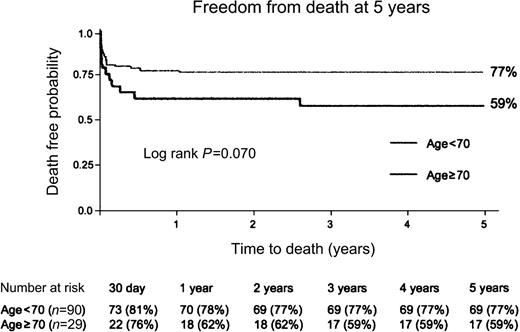
Unadjusted Kaplan–Meier survival estimates are presented in the Fig. 1 . There was no difference in the follow-up time between groups (P=0.6). Patients <70 years had a median follow-up time of 1332 days (range=493–2926) and patients 70 years or older had a median follow-up time of 1505 days (range=514–2845). There was a trend towards a decreased 5-year actuarial survival in patients ≥70 years compared to younger patients, but this difference did not reach statistical significance (Hazard ratio=0.53, 95% Confidence Intervals=0.26–1.07, log-rank P=0.07). Specifically, actuarial 5-year survival was 77% for patients <70 years vs. 59% for those ≥70 years (Fig. 1).
Actuarial 5-year survival curves for the patients groups are presented (Kaplan–Meier survival estimates). P-value, calculated with the log-rank test is reported.
4. Discussion
General aging of the population and improvements in perioperative management have led to an increasing referral of elderly patients for cardiac surgery. Advanced age is considered, by some, a risk factor for operative mortality for emergent repair of type A aortic dissection [9–13]. The decision to operate on elderly patients with type A aortic dissection remains controversial as previous reports have documented an operative mortality rate range in elderly patients between 13% and 46% [14, 15]. Our study showed that acceptable results can be achieved in patients over 70 years undergoing emergent repair of acute type A dissection.
4.1. Principal findings
4.1.1. Operative mortality
We conducted a two-institution observational study to address the impact of advanced age in the outcomes after repair of type A dissection. Based on the findings of our study, emergent repair of type A dissection is justified on patients over 70 years, a finding that supports some authors' findings [14, 16] but conflicts with others [4, 11, 14]. In our series, operative mortality in patients ≥70 years was comparable to that of younger patients (24.1% vs. 18.8%, P=0.60). In our study, there were only three patients >80 years who underwent surgery. Of those three patients, one died 24 days after surgery, the second died 64 days after surgery and the third died 164 days after surgery.
4.1.2. Hemodynamic instability and survival
Hemodynamic instability was associated with dismal prognosis since the operative mortality for those patients was 71.4%, in our study. Our findings agree with the findings of Trimarchi and colleagues [5] who documented a 31.4% in-hospital mortality in unstable patients compared to 16.7% in stable patients (P<0.001). The higher mortality found in our study for unstable patients may be explained by the fact that Trimarchi et al. included patients with neurologic instability such as stroke or coma, as well as patients who presented with mesenteric ischemia or acute renal failure as unstable patients, whereas our study only included patients who presented with hemodynamic instability and/or evidence of myocardial infarction or ischemia as unstable patients [5].
4.1.3. Postoperative morbidity
Early postoperative complications such as stroke, postoperative bleeding and renal failure were comparable between the two groups. Stroke rates in both groups were comparable with those recorded in the literature for type A dissection (20–37.5%) [2, 14, 16]. Elderly patients were more likely to receive a smaller aortic valve prosthesis compared to younger patients. The higher revascularization rate in the elderly population may be explained by the fact that coronary artery disease is more prevalent in older patients. Aortic valve preservation was achieved in the majority of our patients (71%), and root replacement was infrequent (12%), regardless of age. The open distal anastomotic technique was performed for the majority of patients (63%). Forty-five out of 75 patients who had open distal anastomosis underwent hypothermic circulatory arrest without antegrade or retrograde perfusion. The median circulatory arrest time was 26 min, that is considered a safe period of circulatory arrest [17]. Early experience at the Mayo Clinic suggested that 45 min was the maximum safe duration [17]. The portrayal at 18 °C of essentially complete safety of 30 min of circulatory arrest is consistent with all previous studies [17]. The shorter circulatory arrest times (3–5 min) accomplished with the open distal anastomotic technique involved axillary arterial cannulation, inspection of the arch and resection of the ascending aorta under a brief period of circulatory arrest, isolation and clamping the head vessels and then establishing antegrade cerebral perfusion via the axillary artery, while constructing the distal anastomosis.
The reoperations for bleeding rates were relatively higher particularly in younger patients. The rates of reoperation for bleeding after repair of type A dissection are variable in the literature and range between 10% and 40% [2, 15, 18]. The reasons for a higher reoperation for bleeding rate, after type A dissection compared to other cardiac surgery procedures are the fragility of the dissected tissue and persistence of the false lumen after surgery leading to increased wall stress [18].
4.1.4. Actuarial survival
In our series, there was a trend towards a lower 5-year actuarial for patients over 70 years compared to younger patients (79% vs. 59%, P=0.07), however, this trend was not statistically significant. Comparing the two curves, both cohorts of patients had a high risk of death in the first 30 days following surgery but the curve plateaus thereafter for younger patients, while the risk of death in elderly patients continues even after the first 30 days (Fig. 1). A lower 5-year actuarial survival in older patients may also be explained by the fact that older patients have a shorter life-expectancy than younger patients [15].
4.2. Clinical implications
In this study, we used a real-world unselected cohort of patients with prospectively acquired data by dedicated data management personnel to document comparable postoperative outcomes between patients ≥70 years and younger. Based on our results, emergent repair of acute type A dissection should not be denied on the sole basis of age, since the survival without operative intervention is dismal [15]. Hemodynamic instability was associated with an extremely poor prognosis in both age groups which raises the question of whether surgical intervention is warranted in this subset of patients.
4.3. Study limitations
Limitations of the study include the retrospective methodology. The small sample size is another limitation of our study, which did not allow for multivariate analysis of variables between the two groups. Furthermore, the small sample size probably did not allow the trend in a lower 5-year actuarial in patients ≥70 years to reach statistical significance (type II error). The study may also have introduced potential bias by the fact that nine different surgeons from two different institutions operated on the patients. Investigation of the causes of late mortality, late reoperations on the remaining dissected aorta (aortic arch, descending aorta), as well as the fate of the false lumen were beyond the scope of our study and need to be the focus of future reports evaluating the long-term outcome of patients undergoing repair of type A dissection.
In conclusion, emergent repair of type A dissection should not be denied on the sole basis of advanced age. Hemodynamic instability portends a poor prognosis, regardless of age. Expedient referral and intervention might help to improve results by decreasing preoperative dissection-related complications which may lead to hemodynamic instability.
Presented at the 23rd Annual Meeting of the European Association for Cardio-thoracic Surgery, Vienna, Austria, October 18–21, 2009.
References
Conference discussion
Dr. R. Bonser (Birmingham, UK): You add some data to the debate about the provision of emergency surgery in dissection to patients at the extremes of age. In your report, you describe relatively satisfactory outcomes for patients over 70 with a mortality rate of about 20% and a stroke rate similarly about 20%.
Last year there were two very important papers that came out from Nihon University which described their experience with octogenarians and highlighted some very important points.
Firstly, elderly patients who survived dissection but suffer significant morbid events appeared to have a very miserable and limited survival. And there is a question as to the role of surgery at all in this group.
Secondly, in recognizing that the very old patients had a worse outcome even with an ostensibly successful repair, they described a very rapid warm bypass and a rather exaggerated rewarming technique that they felt was demonstrating an improvement in their outcomes.
So my questions to you:
How many of your patients were actually over 80?
The second thing is that you've described quite a high incidence of coronary artery disease. Do you mean coronary artery disease in the patients who were having angiography, or do you mean episodes of coronary malperfusion? If you answer those first.
Dr. Hagberg: I'll take the second question first. That's true coronary artery disease, that's angiography-based.
Dr. Bonser: How many patients underwent angiography?
Dr. Hagberg: Very few.
Dr. Bonser: Okay. So what targeted the angiography?
Dr. Hagberg: I'm sorry?
Dr. Bonser: You said that it was based upon angiography.
Dr. Hagberg: Correct.
Dr. Bonser: And then you said that very few people underwent angiography.
Dr. Hagberg: Very few patients have angiography. I can't tell you exactly how many. It's very surgeon-specific in terms of who gets angiography and who doesn't. I would assume it's based on presenting symptoms and age.
Dr. Bonser: But the data that you presented suggest it's true coronary artery disease?
Dr. Hagberg: Yes.
Dr. Bonser: Okay.
Dr. Hagberg: But you notice that there was no difference in terms of concomitant CABG done between the two groups. And I assume that means that there was more coronary, or at least perceived coronary malperfusion or sheared-off coronaries in the younger patients.
Dr. Bonser: The second query that I'd like to raise was that the usage of deep hypothermic circulatory arrest was only about 60%, which infers that about 40% of the patients were undergoing surgery with a clamp-on technique.
Dr. Hagberg: Correct.
Dr. Bonser: Has that changed with time, or is that a persistent technique in your centers?
Dr. Hagberg: That's a good question. I wish I could change it. My personal strategy is to use deep hypothermic circulatory arrest in all patients. But again, these are 9 different surgeons and everybody has their particular strategy for dealing with this situation, and I guess some surgeons are more comfortable with using the clamp.
Dr. Bonser: Thirdly, when your elderly patients suffered a non-fatal stroke or other serious morbidity, what happened to them in terms of their return to independence, and any data on quality of life?
Dr. Hagberg: I can't answer that question.
Dr. Bonser: And are you considering changing your perfusion and surgical practices in the light of the data from Dr. Hata or in the light of data that you've seen more recently?
Dr. Hagberg: I think that I would do deep hypothermic circulatory arrest, only if I thought that I would have a circulatory arrest time of 20 min or less. If I thought I needed to do extensive arch reconstruction, then I would use selective antegrade cerebral perfusion. That's my personal strategy.
Dr. Bonser: And finally, how many of the patients were actually over 80?
Dr. Hagberg: Three patients, one died and two survived.
Dr. E. Weigang (Mainz, Germany): As I mentioned yesterday regarding our German Registry for Acute Aortic Dissections Type A (GERAADA), we also analyzed several age subgroups. We found no differences among the subgroups except in the cohort over age 80. So my question is: Did you analyze the group aged over 80 years as well and, if so, what are your results?
Dr. Hagberg: Yes, as I just told Dr. Bonser, we only had 3 patients over 80, so it would be impossible to really do any statistically valid comparison. What I'd like to do is get a huge database, like you have, to analyze such questions. In fact, Dr. Bavaria and I were talking last night in terms of trying to start exactly what you have (and you presented yesterday) in the USA through the STS database.
Dr. Weigang: We have experienced the same in our own institution. We have very good results in younger patients, and have observed no significant differences among the different age groups, but we do have a significantly higher mortality rate in patients over 80.
Dr. M. Shrestha (Hannover, Germany): I've got a question. The question about whether we should be operating on patients above 70, in our view, at least from Hannover point of view, is false. Because if we do not operate on them, they are going to die anyway. So we published our results two years back, and we do see a difference between mortality in patients below 70 and above 70. And at that time we found that in patients below 70, if they are stable, of course, the mortality was around 15%; but if they are above 70, it is higher, up to almost 28–29%. But the thing is that if you do not operate, they do die. So our policy has been that if the patients reach Hannover Medical School, they would be operated, everyone, regardless of their age. Because we should not refuse operation to anyone only on the basis of age.
And the second point is that I think these patients, the older patients, you cannot do total arch or valve repair, because long cross-clamp times and circulatory arrest do have a point in increasing the mortality. So maybe it would be easier to do a valve replacement, if the valve is involved in these patients. And one should not refuse patients just because they are above 70.
Dr. Hagberg: I'm not sure I understood the whole question, but I think it –.
Dr. Shrestha: No, it was not a question. It was a point that we should not refuse patients just because they are old.
Dr. Hagberg: I agree with that. However, if you have a patient that's 80 years of age or older and they come in with a creatinine of 2 and they're in shock and they have an ascending aortic aneurysm, they have an arch aneurysm, and they have a descending thoracic aortic aneurysm and they have acute dissection, then you have to think long and hard about operating on that patient.
Dr. Shrestha: Our policy, since the time of Hans Borst, has been anyone who comes in alive gets an operation.
Dr. U. Tesler (Milan, Italy): Would you consider severe preoperative neurological damage a contraindication to repair of an acute aortic dissection? Have you done any study on that?
Dr. Hagberg: We have not done a study on that. I personally operate on those patients hoping that they will turn around.