Abstract
Blastocystis hominis has been reclassified as a protozoan parasite. Its role as a human pathogen is somewhat controversial. There has been a dramatic increase in the frequency of B. hominis infection in association with diarrhea especially in immunocompromised hosts like AIDS patients, travelers, homosexuals, day care children, animal handlers especially zoo keepers, etc. Recent reports suggest that B. hominis is an emerging pathogen; hence, we have undertaken this study to detect B. hominis from stool samples of patients attending our hospital. About 200 stool samples were tested by light microscopic examination, for observing wet mounts with saline and Lugol’s iodine. Permanent staining of fecal smear by Gram’s staining and modified acid fast staining was done. The stool sample which was microscopically positive for B. hominis was cultured on Lowenstein–Jensen’s (LJ) medium. In one patient, the vacuolated form of B. hominis was observed in wet mount with saline preparation of stool sample. This was very clearly seen in wet mount with Lugol’s iodine. In Gram’s stained preparation, also the vacuolated form was observed. Detection of B. hominis was not possible by modified acid fast staining. B. hominis was also grown on LJ medium which is an egg-containing medium. Clinical microbiology laboratories should start screening of stool samples for B. hominis as it is an emerging pathogen.
Similar content being viewed by others
Introduction
Taxonomically controversial intestinal protozoal pathogen, Blastocystis hominis, remains somewhat mysterious due to its pleomorphism, its uncertain taxonomic position, and its pathogenesis. B. hominis was discovered in 1870 by the Russian physician Fedor Aleksandrovich Lesh (Lesh 1975).
Previously, B. hominis was considered as a yeast. But in year 1967, Zierdt et al. reported it as protozoa by its ultrastructural characteristics and considered it an intestinal protozoal parasite. Classification of B. hominis is given as follows: subkingdom—Protozoa, subphylum—Sarcodina, and suborder—Blastocystina.
Still, its exact taxonomical position remains undetermined (Sukthana 2001).
Morphology
Numerous morphological forms have been described, but three forms which are commonly found are (a) vacuolar: found commonly in fecal specimen, (b) granular: found in culture, and (c) amoeboid: found only in patient with acute diarrhea (Sukthana 2001). Recent studies revealed that other forms such as cystic, avacuolar, and multivacuolar are also observed (Singh et al. 1995; Vdovenko 2000). The vacuolated form is the most common and variable in size (5–30 μm and usually 8–10 μm), and each consists of a central vacuole surrounded by a thin rim of cytoplasm containing four to six nuclei. The vacuolated form can be stained by Trichrome stain and the vacuole appeared red to blue and four to six nuclei in the peripheral cytoplasmic rim are stained purple. The amoeboid form develops pseudopodia like amoebae.
Mode of transmission
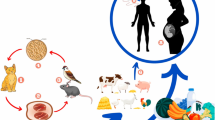
The transmission occurs mainly by feco-oral route among the human beings, i.e., from one person to another. Adults are more commonly infected. Zoonotic transmission is also reported in animals like chicken, pig, and horse which are responsible for the transmission of infection to human (Yoshikawa et al. 1996).
Life cycle
There are two types of life cycles described, asexual by binary fission and sexual by autogamy to form primary cysts. There are two forms of cysts: thick-walled resistant form and thin-walled cyst responsible for autoinfection (Stenzel et al. 1991).
The life cycle of B. hominis can be described as follows:

Pathogenesis
The role of B. hominis as a human pathogen is controversial. The exact mechanism by which it produces diarrhea is not yet known. B. hominis is most commonly associated with diarrhea in immunocompromised hosts like HIV-infected persons, homosexuals, travelers, day care children, animal handlers, mentally retarded persons, etc. (Carbajal et al. 1997).
The patient mostly present with diarrhea, nausea, abdominal pain, discomfort, and flatulence and may be diagnosed wrongly as irritable bowel syndrome (IBS). The patient may present with fever, eosinophilia, anorexia, fatigue, and nonspecific gastrointestinal symptoms.
With this background, we have tested the stool samples of patients attending Acharya Vinoba Bhave Rural Hospital, Wardha (India) with the aim to detect B. hominis infection in patients from January to June 2011.
Materials and methods
Around 200 stool samples were observed by both direct wet mount preparation with saline and iodine and permanent staining techniques like Gram staining and modified Ziehl–Neelsen staining (Parija 2009).
The stool samples were cultured on blood agar and MacConkey’s agar to isolate any bacterial pathogen. In addition, the stool sample which was microscopically positive for B. hominis was also cultured on Lowenstein–Jensen’s medium, and from the same patient, three stool samples on three different days were collected and processed accordingly. A LJ medium bottle was tightly screw-capped after inoculation. No anaerobic condition could be provided. The saline wet mount preparation from growth, obtained on LJ medium, was observed, after 48 h under ×40 objective of a simple microscope.
Observation
Out of the 200 stool samples observed, only one patient was positive for B. hominis. Three subsequently collected stool samples on three different days showed same consistent finding.
The saline and iodine wet mount preparation of stool sample showed a vacuolar form of B. hominis typically consists of a central body or vacuole surrounded by a thin rim of cytoplasm containing up to six nuclei and the size was approximately 8–10 μm (Figs. 1 and 2) and no other parasitic infection was detected in that stool sample. Gram’s staining was also positive for vacuolar form of B. hominis showing oval to spherical structure with four to five peripheral nuclear structures and central clear space (Fig. 3). The size was approximately 8–10 μm.
The same stool sample which was positive for vacuolar form of B. hominis microscopically was cultured on LJ media. After 48 h of incubation, white very shiny mucoid type of growth appeared. The saline wet mount preparation from growth obtained on LJ medium showed a granular form of cyst of B. hominis (Figs. 4 and 5). On blood agar and MacConkey’s agar, Escherichia coli was isolated repeatedly which might be a commensal.
The patient was then treated with metronidazole orally 400 mg thrice daily for 7 days and on follow-up and was completely recovered and not having any symptoms. B. hominis infection is usually treated with metronidazole 250–700 mg thrice daily for 7 days or 2 g per day for 5 days.
Discussion
The patient, 22-year-old female and a Nursing student, was a resident of a nearby village. She was HIV seronegative. The patient had history of constipation and diarrhea alternately for the last 6 months, with recent passage of fresh blood in stool. The patient had also history of nonspecific symptoms like weakness, malaise, anorexia, etc. The patient was already treated with co-trimoxazole, furazolidone, norfloxacin, ciprofloxacin, etc. and antacid like Gellucil and even H1 receptor blocker, e.g., ranitidine, and complete HCl synthesis blocker omeprazole, etc. by different physicians from time to time but the symptoms persisted. Due to highly genotypic polymorphism, development of a genetic marker will help detect B. hominis. Culture is also not feasible for most of the clinical microbiology laboratory. Scanning electron microscopy has shown that its outer coat has a fibrillar structure and ultrastructurally electron dense material may be present in the central vacuole which may be because of the accumulation of lipid.
The concentration methods should not be employed for observation of B. hominis as they will destroy cell morphology (Sukthana 2001). Also, for preservation and fixation of B. hominis, refrigeration should not be done as it destroys the cyst form of the parasite. The vacuolar form of B. hominis observed under saline and iodine preparation is quite characteristic. The differential diagnosis with cysts of Entamoeba histolytica, Entamoeba hartmanni, Endolimax nana, Cryptosporidium, macrophages, RBCs, polymorphonuclear leukocytes, yeast cells, etc. has to be done morphologically.
The stool sample which was microscopically positive for the vacuolated form of B. hominis was cultured on Lowenstein–Jensen’s medium also. This was because different studies had reported that egg-containing media should be used for the cultivation of B. hominis(Zamn et al. 1995) and usually modified whole egg slant medium with Lock’s solution overlay with 30 % horse serum is used (Zierdt 1991). Other media used are Pavlova’s medium using human serum along with potasic G penicillin and streptomycin (Zerpa et al. 2000), Boeck Drhohlar NIH modified medium with antibiotics, and liquid medium for cultivation which requires Lock’s solution containing sodium bicarbonate (0.8 g), potassium phosphate (0.6 g), glucose (5 g), sodium chloride (16 g), calcium chloride (0.4 g), potassium chloride (0.4 g), magnesium chloride (0.02 g), and sodium phosphate (4 g) (Lanuza et al. 1997). We actually did not have any egg-containing media for parasitic culture, so we used LJ media to see whether B. hominis could be grown or not. To our surprise, saline wet mount preparation of growth on slant of LJ media showed the characteristic granular form and cyst form of B. hominis (Figs. 4 and 5).
Infection of B. hominis can be controlled by incorporating good personal hygiene, improvement in sanitary conditions, education about prevention of fecal contamination of environment, and proper washing of fruits or other contaminated material before eating.
To conclude, we must say that though B. hominis pathogenicity is controversial, all stool samples should be screened for B. hominis, and if detected, patient should be treated accordingly for B. hominis if no other pathogens are detected.
References
Carbajal JA, Villar J, Lanuza MD et al (1997) Clinical significance of Blastocystis hominis infection: epidemiologic study. Med Clin 108:608–612
Lanuza MD, Carbajal JA, Villar J et al (1997) Description of an improved method for Blastocystis hominis culture and axenization. Parasitol Rev 83:60–68
Lesh FA (1975) Massive development of amebas in the large intestine. Am J Trop Med Hyg 24:383–392
Parija SC (2009) Common laboratory methods in parasitology. In: Textbook of medical parasitology, 3rd edn. All India Publishers and Distributors, Chennai, pp 360–370
Singh M, Suresh K, Ho LC et al (1995) Elucidation of the life cycle of the intestinal protozoan Blastocystis hominis. Parasitol Res 81:446–450
Stenzel DJ, Boreham PF, McDougall R (1991) Ultrastructure of Blastocystis hominis in human stool samples. Int J Parasitol 21:807–812
Sukthana Y (2001) Is Blastocystis hominis a human pathogenic protozoan? J Trop Med Parasitol 24:16–22
Vdovenko AA (2000) Blastocystis hominis: origin and significance of vacuolar and granular forms. Parasitol Res 86:8–10
Yoshikawa H, Nagono I, Yap EH et al (1996) DNA polymorphism revealed by arbitrary primers polymerase chain reaction among Blastocystis hominis strains isolated from humans, a chicken, and a reptile. J Eukaryot Microbiol 43:127–130
Zamn V, Howe J, Ng M (1995) Ultrastructure of Blastocystis hominis cysts. Parasitol Res 81:465–469
Zerpa LR, Huicho L, Naquira C et al (2000) A simplified culture method for Blastocystis hominis. Rev Mex Patol Clin 47(1):17–19
Zierdt CH (1991) Blastocystis hominis—past and future. Clin Microbiol Rev 4:61–79
Acknowledgments
The authors highly acknowledge the help rendered by the Datta Meghe Institute of Medical Sciences, Wardha.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Basak, S., Rajurkar, M.N. & Mallick, S.K. Detection of Blastocystis hominis: a controversial human pathogen. Parasitol Res 113, 261–265 (2014). https://doi.org/10.1007/s00436-013-3652-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-013-3652-4