-
PDF
- Split View
-
Views
-
Cite
Cite
Sanjay Sethi, Moxifloxacin for the Treatment of Acute Exacerbations of Chronic Obstructive Pulmonary Disease, Clinical Infectious Diseases, Volume 41, Issue Supplement_2, July 2005, Pages S177–S185, https://doi.org/10.1086/428058
Close - Share Icon Share
Abstract
Background/purpose. The significant impact of acute exacerbations of chronic obstructive pulmonary disease (AECOPD) is now recognized. This recognition has led to increased efforts to provide evidence-based, appropriate treatment of AECOPD, to minimize its negative impact. This article reviews the bacterial etiology of AECOPD and clinical trials (both placebo-controlled and antibiotic comparison trials) that support the use of antibiotics for AECOPD, with an emphasis on the role of newer fluoroquinolones for the treatment of patients with this condition. A discussion of patient stratification that permits identification of those who require initial aggressive antibiotic therapy is presented.
Main findings. Among the treatment modalities for exacerbations, the role and choice of antibiotics is hotly debated. Current evidence supports the use of antibiotics in the treatment of AECOPD, because bacterial pathogens cause approximately half the exacerbations, and because empirical antibiotics have a significant benefit in most exacerbations. Several recent investigations have aided in the development of a rational antibiotic strategy for AECOPD. These include outcome studies that have identified patients who are likely to have a poor outcome of their exacerbation and, therefore, are candidates for aggressive initial antibiotic therapy. Studies of the new fluoroquinolone agents have shown superior short- and long-term clinical results among patients with AECOPD who are at risk of a poor outcome.
Conclusions. Theoretical concerns about the emergence of resistance to the fluoroquinolones dictate not only the appropriate use of these drugs but, also, the use of the most-potent agents available in this class, to sustain their usefulness over time. Such selected use of the new fluoroquinolones balances individual benefit with societal concerns regarding the use of these agents for the treatment of AECOPD.
Chronic obstructive pulmonary disease (COPD) is a global disease that is expected to become increasingly prevalent in the coming decades [1]. It is estimated that the prevalence of diagnosed cases of COPD in the United States is ∼10 million, with an equal number of undiagnosed cases [2]. A cardinal feature of COPD is the occurrence of intermittent flare-ups of symptoms, comprising ≥1 of the following: increased dyspnea, sputum production, and sputum purulence [3]. These intermittent acute exacerbations of COPD (AECOPD) were long thought to be of little consequence, primarily because older studies suggested that these episodes did not contribute to the progressive loss of lung function, which is another cardinal feature of COPD [4]. In the past decade, studies have refuted these earlier contentions by showing that, not only do exacerbations contribute to the loss of lung function, but they also have a major effect on the quality of life of these patients and account for 35%–45% of the costs of caring for patients with COPD [5,6–7]. Recognition of the significance of these exacerbations has led to increased efforts to provide evidence-based, appropriate treatment, to minimize their negative effects [8,9–10].
Among the treatment modalities for exacerbations, the role and choice of antibiotics is hotly debated [11, 12]. Whether antibiotics are indicated in the treatment of AECOPD would depend on (1) whether bacteria cause exacerbations and (2) whether studies show improved outcome with antibiotics, compared with placebo. As discussed below, several recent studies clearly implicate bacteria as a cause of 40%–50% of exacerbations. This evidence does provide a rationale for the use of antibiotics in the treatment of exacerbations; however, it does not automatically imply that antibiotics will provide a benefit. Placebo-controlled prospective trials and retrospective data have shown such benefit of antibiotics. The influence of study design on the results of such trials needs to be understood to properly interpret the sometimes contradictory results of these trials.
Another controversial issue is whether the choice of an antibiotic has any influence on the clinical outcome of exacerbations. It seems logical that antibiotics with superior in vitro antimicrobial activity would be associated with a superior clinical outcome of AECOPD, compared with agents that now have limited antimicrobial activity because of emerging antibiotic resistance. However, most antibiotic comparison trials have not shown such clinical superiority [13]. On the contrary, recently published data about potent new fluoroquinolones (e.g., moxifloxacin and gemifloxacin) have shown superior outcomes with these agents [14, 15]. A careful analysis of clinical trials demonstrates how study design, especially inclusion criteria and choice of end points, determines the results of antibiotic comparison trials in AECOPD [16]. Studies of new fluoroquinolones that show improved short- and long-term outcomes have used improved study design and have included clinically relevant but unconventional end points.
The enthusiasm for widespread use of the fluoroquinolones in the treatment of exacerbations of COPD has to be tempered by concerns about emerging resistance to these agents among pathogens implicated in these infections, specifically Streptococcus pneumoniae and Pseudomonas aeruginosa [17, 18]. It is, therefore, appropriate that these agents be used in patients who would derive the maximum benefit from early aggressive antibiotic therapy. Recent observational studies have clearly identified easily assessable clinical characteristics that, at the time of an exacerbation, can identify patients who are either at risk for a poor outcome or are at increased risk for infection with drug-resistant pathogens. Such patients, identified as having “complicated chronic bronchitis,” are appropriate candidates for early aggressive antibiotic therapy with such agents as the potent new fluoroquinolones [10].
Another important consideration is whether the specific choice of an agent among a class of antibiotics, such as the fluoroquinolones, is of any consequence. Although clinical differences in outcome among these agents are likely to be small, an important concern is the potential for emergence of resistant clones with the use of less-potent agents. An important determinant in the emergence of resistance is the pharmacokinetic/pharmacodynamic (PK/PD) relationship between an antibiotic and a pathogen [19]. These PK/PD considerations suggest that the use of more-potent agents in a class of antibiotics is prudent and will preserve the therapeutic efficacy of the class of drugs for a longer period [18, 20].
Bacterial Etiology Of Aecopd
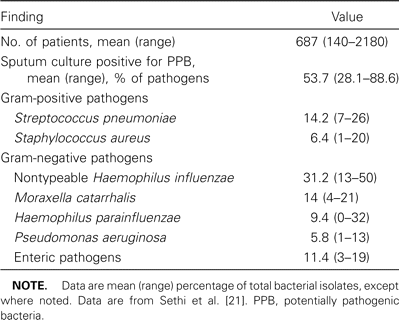
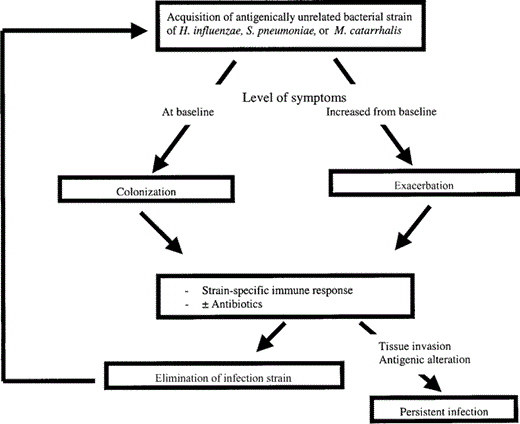
Bacterial pathogens are recovered in sputum in 40%–50% of exacerbations, and serological data implicate Chlamydophila pneumoniae infection in 5%–10% of the episodes (table 1) [22]. Whether the isolation of bacteria from sputum is related to the occurrence of AECOPD or represents isolation of colonizing species that are “innocent bystanders” has been a contentious issue for decades [11, 23]. Recent research using new investigative techniques, including bronchoscopic sampling of the airways, molecular epidemiology of bacterial pathogens isolated from sputum, immunological assays, and measurement of inflammatory markers, has clearly established that bacteria indeed cause a substantial proportion of exacerbations of COPD (figure 1). In-depth discussion of the results of these studies can be found elsewhere [22, 24].
Bacterial pathogens isolated from sputum in recent studies of acute exacerbations of chronic bronchitis.
Proposed mechanism of bacterial infection of chronic obstructive pulmonary disease. H. influenzae, Haemophilus influenzae; M. catarrhalis, Moraxella catarrhalis; S. pneumoniae, Streptococcus pneumoniae.
Although the role of bacteria in 40%–50% of episodes AECOPD is now beyond doubt and would support the use of antibiotics to treat exacerbations, evidence for bacterial causation does not automatically imply that antibiotic therapy will be beneficial. In AECOPD, as with other mucosal infections, such as acute otitis media and acute sinusitis, there is a substantial rate of spontaneous resolution because of the immune-inflammatory response to infection. However, spontaneous resolution in AECOPD is not very efficient. In the largest placebo-controlled trial to date, spontaneous resolution was observed in only 55% of patients at 3 weeks after the onset of infection [3]. Therefore, a substantial proportion of exacerbations do not resolve spontaneously, even over a relatively long time. Therapy to resolve symptoms to baseline levels (i.e., the preexacerbation period) is therefore indicated. Antibiotic therapy could also hasten the resolution of the symptoms of exacerbation, compared with spontaneous resolution. Faster resolution could have additional benefits, including less time lost from work or usual activities. Rapid resolution of inflammation could also result in a decreased proteolytic load in the airways and, consequently, less lung damage from the inflammatory process of the exacerbation [25].
Another confounding issue in the treatment of exacerbations with antibiotics has been the suggestion that treating the inflammatory response per se in AECOPD with oral corticosteroids is adequate. To date, in only one trial involving patients with AECOPD have all the patients received steroids and been randomized to receive antibiotics or placebo [26]. Although that study did not show a benefit of antibiotic use, several limitations of that study, as discussed below, make it uncertain that oral corticosteroids alone are adequate treatment of exacerbations. In conclusion, in spite of the confirmation of bacterial infection as an important cause of AECOPD, placebo-controlled antibiotic trials are necessary to provide direct evidence that antibiotics are of significant benefit in the treatment of exacerbations.
Placebo-Controlled Antibiotic Trials
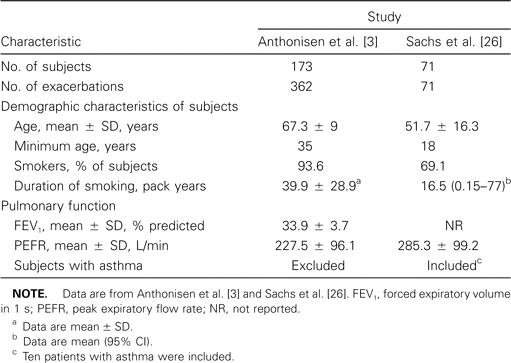
Compared with the number of antibiotic comparison trials (see below), relatively few placebo-controlled trials have been performed in AECOPD. In fact, a meta-analysis published in 1995 was only able to identify 9 trials of adequate quality to be included in the analysis [27]. A small but significant beneficial effect of antibiotics over placebo for acute exacerbation was demonstrated in this analysis [27]. Among these trials, the largest and most widely quoted trial was conducted by Anthonisen et al. [3] in the early 1980s. A total of 362 exacerbations were included in that study, and patients were randomized to receive placebo or 1 of the following antibiotics: amoxicillin, trimethoprim-sulfamethoxazole (TMP-SMZ), or doxycycline. Antibiotic therapy was superior to placebo for the primary end point (clinical resolution or improvement of symptoms at 3 weeks after enrollment), with 68% of patients achieving clinical success with antibiotics, compared with 55% of patients receiving placebo (P < .01) [3]. Although that trial was a landmark study, it had several limitations. Corticosteroid therapy was not controlled or stratified, although equivalent proportions (∼43%) of patients in both study arms received corticosteroids. The end point was obtained at 3 weeks after enrollment, which, although consistent with regulatory agency requirements, is not as clinically relevant as measurements of efficacy made at 3–5 days after the start of treatment. Most physicians and patients would contemplate a change in therapy or additional therapy if sufficient resolution of symptoms has not occurred at this time, rather than wait 3 weeks. In addition, microbiological analysis was not included in this study; thus, the benefit of antibiotics for confirmed bacterial infections alone could not be assessed.
Subsequent to the meta-analysis [27], the findings of 3 additional placebo-controlled trials have been published [26, 28, 29]. An analysis of these studies illustrates the difficulties in the interpretation of placebo-controlled studies of antibiotic therapy for acute exacerbation because of the heterogeneity in patient populations, antibiotics, concomitant treatment, and end points. A widely quoted study conducted by Sachs et al. [26] has the distinction of being the only placebo-controlled trial of antibiotic therapy for AECOPD in which a short course of oral corticosteroids was administered to all patients. In that study, no benefit was seen with antibiotics, with a clinical success rate of 91% in both the placebo and antibiotic groups. The conflicting results of the studies by Sachs et al. [26] and Anthonisen et al. [3] could be partially attributable to the use of corticosteroids in the former study. However, significant differences in the patient populations included in the 2 studies are likely more important (table 2). In fact, with the study design of the investigation by Sachs et al. [26], to see a benefit with antibiotics would have been a surprise.
Comparison of study design and patient characteristics of 2 placebo-controlled trials of antibiotics in acute exacerbation of chronic obstructive pulmonary disease.
In another recent placebo-controlled trial, Nouira et al. [28] enrolled 93 patients with exacerbations severe enough to require ventilatory support in an intensive care unit and randomized them to receive ofloxacin or placebo. No systemic corticosteroids were administered. Bacterial pathogens were isolated in tracheobronchial aspirates obtained from 61% of patients. Antibiotics reduced mortality and the need for additional antibiotics by 17.5- and 28.4-fold, respectively.
Allegra et al. [29] recently published a reanalysis of an older trial that was originally published in 1991. The new analysis compared the use of amoxicillin-clavulanate with that of placebo for 414 exacerbations and determined the effect of forced expiratory volume in 1 s (FEV1) on outcome. The clinical success rate was significantly superior with amoxicillin-clavulanate (86.4%), compared with placebo (50.6%) (P < .001). A greater benefit of antibiotic therapy was associated with increasing severity of underlying airway obstruction. A careful examination of the placebo-controlled trials of antibiotic therapy for AECOPD demonstrates that antibiotics have benefit, especially if patients are enrolled in adequate numbers, have moderate-to-severe underlying COPD, or if the primary end point is determined early during the course of the exacerbation [3, 28, 29].
Antibiotic Comparison Trials And Aecopd
A majority of evidence regarding AECOPD now supports the bacterial etiology of exacerbations and the benefit associated with antibiotics for all but mild (single-symptom) episodes. However, there remains considerable disagreement as to whether the choice of antibiotic has any influence on the outcome of exacerbations. Specifically, there is disagreement as to whether the use of an antibiotic with excellent in vitro microbiological activity results in a better outcome of AECOPD than does an antibiotic with limited in vitro microbiological activity. Until recently, there was no evidence that the choice of antibiotics influenced the outcome of acute exacerbations. In fact, antibiotic comparison trials designed to show equivalence between agents for registration purposes did not demonstrate clinical superiority of any one class of antibiotics over another [13].
Recent guidelines recommend the use of amoxicillin, TMP-SMZ, and doxycycline for treatment of patients with AECOPD [8]. This recommendation is based on results of antibiotic comparison trials and on the fact that these antibiotics demonstrated efficacy in the trial of Anthonisen et al. [3]. This recommendation, although evidence based, ignored the fact that the recommended agents now have limited in vitro activity against the major pathogens implicated in AECOPD (e.g., S. pneumoniae, nontypeable Haemophilus influenzae, and Moraxella catarrhalis), because of the emergence of resistance in these pathogens. In addition, increasing longevity of patients with severe obstructive pulmonary disease and selective antibiotic pressure has led to increasing recognition that relatively resistant bacterial species, including P. aeruginosa and other gram-negative Enterobacteriaceae, are etiologic agents of AECOPD.
During the past few years, evidence of the clinical implications of emerging in vitro resistance has accumulated from retrospective studies and prospective trials of AECOPD. In a retrospective analysis, patients with AECOPD treated in an emergency department were assessed for relapse within 14 days [30]. Patients treated with antibiotics had a significantly decreased relapse rate (from 32% to 19%), compared with patients not treated with antibiotics (P < .001). In addition, patients treated with amoxicillin had a relapse rate even higher than that of patients not given antibiotics (54% vs. 32%; P = .006). This analysis demonstrated that, when antibiotics are clinically indicated, use of an agent with limited in vitro activity against the likely pathogens is associated with a suboptimal outcome.
Another retrospective analysis of patients treated for AECOPD revealed that the use of a third-line agent (amoxicillin-clavulanate, azithromycin, or ciprofloxacin) was associated with fewer clinical failures than was use of first-line agents (amoxicillin, co-trimoxazole, tetracycline, or erythromycin) (clinical failure rate, 7% vs. 19%) [31]. In addition, third-line agents were associated with a significantly lower incidence of hospitalization (5.3% vs. 18%; P < .02) and a longer time to the next exacerbation than were first-line agents (P < .05).
In a recent placebo-controlled randomized trial of oral corticosteroids for AECOPD, all patients received antibiotics, but the choice of antibiotics was confined to agents with limited antimicrobial activity (TMP-SMZ and doxycycline) [32]. Patients in the placebo arm of the study (i.e., those who did not receive corticosteroids) who received only the antibiotics had a clinical success rate of 57%, similar to the spontaneous resolution rates seen in the study by Anthonisen et al. [3], which was performed 2 decades earlier, before the emergence of resistance to these agents. Even in the group randomized to receive corticosteroids plus antibiotics, the clinical success rate of 73% was much lower than those seen in contemporary antibiotic trials [15, 33] that used more-potent antibiotics, such as the fluoroquinolones. These remarkably low clinical success rates are potentially attributable to the limited in vitro activity of the antibiotics used. However the absence of confirmed bacteriological data in this study does not allow a firm conclusion.
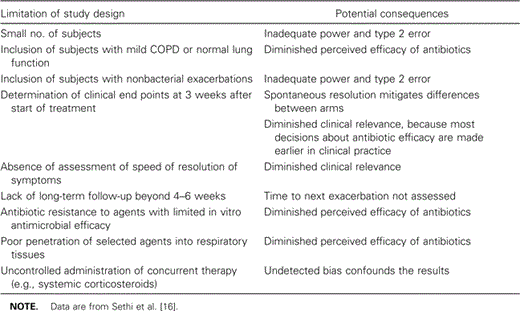
Why have antibiotic comparison trials of AECOPD shown clinical equivalence, in spite of obvious differences among the agents, with regard to in vitro activity and in vivo microbiological efficacy? Several reasons for such equivalence can be identified on the basis of limitations of design in these trials (table 3). Recently, however, 2 trials have recognized the limitations of earlier antibiotic comparison trials and, because of better trial design, have been able to show significant differences in outcome of AECOPD between different antibiotic classes.
Limitations of study design and potential consequences of the results of antibiotic trials of acute exacerbations of chronic obstructive pulmonary disease (COPD).
The GLOBE (Gemifloxacin and Long Term Outcome of Bronchitis Exacerbations) trial was a prospective, double-blind, randomized trial in which patients were randomized to receive a new fluoroquinolone (gemifloxacin) or a macrolide (clarithromycin) [33]. There was no statistically significant difference in the 2 treatment groups on the basis of clinical outcome (85.4% for gemifloxacin vs. 84.6% for clarithromycin) at both the standard end-of-therapy assessment (at 10–14 days) and the long-term follow-up assessment (at 28 days). However, bacteriological success was significantly higher with gemifloxacin (81.8%), compared with clarithromycin (62.0%), at the follow-up visit at 4–5 weeks (95% CI, 2.2–37.5) [14]. Subsequently, patients who had a successful outcome at 28 days after therapy were enrolled in a follow-up observation, for a total of 26 weeks. During this surveillance period, the primary outcomes were the frequency of repeat exacerbations, hospitalizations for respiratory disease, and health-related quality-of-life measures. Gemifloxacin was associated with a relative risk reduction of 30% for recurrence of exacerbation, with 71% of the gemifloxacin-treated patients remaining free of recurrence at 26 weeks, compared with 58.5% of patients in the clarithromycin group (P = .016). The rate of hospitalization for respiratory tract illness during the 26 weeks was also lower in the gemifloxacin group than in the clarithromycin group (2.3% vs. 6.3%; P = .059) [33]. Patients who experienced a recurrent exacerbation during the 26 weeks had a lesser improvement in their health-related quality of life, compared with those who remained free of recurrence [34].
The MOSAIC (Moxifloxacin Oral tablets to Standard oral antibiotic regimen given as first-line therapy in out-patients with Acute Infective exacerbations of Chronic bronchitis) trial is a large double-blind study in which 730 patients were randomized to receive a new fluoroquinolone (moxifloxacin) or standard therapy (i.e., amoxicillin, cefuroxime, or clarithromycin) [15]. This trial had several unique features in addition to its relatively large size. The patients enrolled were well characterized, and a substantial proportion had ≥1 risk factor that would predispose to a poor outcome, as discussed below. In addition, patients were enrolled when their COPD was stable, to establish a baseline so that clinical improvement and resolution after treatment of exacerbation could be determined. Randomization to receive an antibiotic took place if patients had an exacerbation that met predefined criteria during the 12 months after enrollment, and the patients were followed up for 9 months after randomization.
Clinical success (resolution and improvement) was equivalent with moxifloxacin and standard therapy (88% vs. 83%) at 7–10 days after the end of therapy. However, moxifloxacin was associated with superior rate of clinical cure (resolution of symptoms to baseline), compared with standard treatment (71% vs. 63%; 95% CI, 1.4–14.9) 7–10 days after the end of therapy, as well as with superior bacteriologic response (91.5% vs. 81%; 95% CI, 0.4–22.1) [15]. Furthermore, when other a priori unconventional end points were examined, moxifloxacin was associated with less requirement for additional antibiotics (8% vs. 14%; P < .01) and an extended time to the next exacerbation (131 vs. 104 days; P = .03) (figure 2) [15].
Life-table analysis of the time to the first composite event (treatment failure, and/or new exacerbation, and/or any further antibiotic treatment), stratified according to the time of the last acute exacerbation of chronic bronchitis (AECB) prior to randomization. Reproduced with permission from the American College of Chest Physicians [15].
Retrospective studies and the GLOBE and MOSAIC trials demonstrate that in vitro microbiological superiority of antibiotics does translate to greater in vivo effectiveness in treating patients with acute exacerbation. Although differences between antibiotics may not be perceptible for the standard regulatory end point of clinical success at 7–14 days after the end of therapy, differences are perceptible when clinically relevant end points—including speed of resolution, need for additional antimicrobials, and time to the next exacerbation—are considered [15, 34].
Stratification In Selection of Antibiotics For Aecopd
COPD is a heterogeneous disease, and acute exacerbations are of varying etiology and severity. A single strategy for antibiotic therapy for all cases of AECOPD is therefore likely to lead to inadequate or excessive antibiotic treatment for many patients. A rational approach to the use of fluoroquinolones for AECOPD is to use them as the drugs of choice for the treatment of certain subgroups of patients with acute exacerbation [13]. Three such groups of patients, who would benefit from initial therapy with a fluoroquinolone, can be identified. The first group includes patients who are at risk of a poor outcome of exacerbation and failure of antimicrobial treatment. Identifiable risk factors for poor outcome include older age (>65 years), underlying severe obstructive lung disease (FEV1, <50%), frequent (>3) exacerbations in the previous year, and the presence of comorbid illness (especially cardiac disease) [10]. The use of antibiotics with the best in vitro activity and excellent PK characteristics, such as the new fluoroquinolones, as first-line therapy is justified for patients with ≥1 of these risk factors. The expected benefit to the patient of improved outcome justifies the higher acquisition cost of the medicines and societal concerns about the emergence of resistance. The second group includes patients who are at increased risk of infection with an antibiotic-resistant pathogen, especially drug-resistant S. pneumoniae. Patients with AECOPD who are at risk of infection with drug-resistant pneumococci are likely to be similar to patients with community-acquired pneumonia, and their risk factors include older age, comorbid conditions, recent (<3 months) therapy with an antibiotic, and immunosuppression [35]. The third group includes patients who are admitted to an intensive care unit for an acute exacerbation. These patients have underlying severe COPD, and, in this population, Enterobacteriaceae and Pseudomonas species have been frequently implicated as a cause of the acute exacerbation [36]. This justifies the use of fluoroquinolone monotherapy as initial therapy for these patients until culture data become available.
Choosing Among The Fluoroquinolones For The Treatment Of Aecopd
Considerations in choosing a particular fluoroquinolone to treat AECOPD would include potent in vitro activity against the major pathogens anticipated, results of clinical trials, the adverse effects profile, and the potential for the emergence of resistance in key pathogens. Among the fluoroquinolones, improved in vitro activity against S. pneumoniae would dictate the use of one of the respiratory fluoroquinolones (e.g., moxifloxacin, gatifloxacin, gemifloxacin, or levofloxacin) in the treatment of AECOPD, rather than one of the older agents (e.g., ciprofloxacin or ofloxacin) [13]. An exception to this would involve the subgroup of patients who require intensive care, as discussed above, for whom ciprofloxacin would represent a better initial empirical choice [10].
Although there are differences among the fluoroquinolones with regard to in vitro activity, differences in the clinical outcomes associated with the respiratory fluoroquinolones that reflect these differences are likely not to be demonstrated, unless very large trials are done. However, differences in in vitro activity and in PK/PD properties among these agents should make us cautious about extending the findings for one agent to other agents [37]. Therefore, the benefits observed with moxifloxacin in the MOSAIC trial and with gemifloxacin in the GLOBE trial should not automatically be extended to the other agents. Although some adverse effects (e.g., QT interval prolongation) of the respiratory fluoroquinolones are shared as class effects, others appear to be related to individual agents. Because many patients with AECOPD are elderly and could have diabetes, the increasing reports of disturbances in glucose metabolism related to gatifloxacin are a concern [38].
Resistance of S. pneumoniae to the fluoroquinolones has been reported to be especially high in patients with COPD [39]. This is to be expected, because these patients have high bacterial loads in their airways, have repeated infections, and have received repeated courses of antimicrobials. The antibacterial effects of the fluoroquinolones are related to interference with the actions of 2 enzymes involved in bacterial DNA replication—DNA gyrase and topoisomerase IV. Point mutations resulting in single amino acid substitutions in topoisomerase IV or in DNA gyrase result in the development of resistance to the fluoroquinolones [40,41–42]. The development of significant resistance (exceeding the achievable serum level of the drug) to the new fluoroquinolones (e.g., moxifloxacin, gatifloxacin, and gemifloxacin) in S. pneumoniae requires >1 mutation [40,41–42]. Theoretically, this requirement, along with the favorable PK/PD properties of these agents, should delay the emergence of resistance to fluoroquinolones if the use of these agents is preferred to the use of less-potent agents (figure 3) [18,19–20]. Although this hypothesis is unproven, it seems to be an important reason, from a societal perspective, to prefer these agents over levofloxacin for the treatment of patients with AECOPD.
Area under the inhibitory curve (AUIC) for fluoroquinolones used to treat respiratory infections due to Streptococcus pneumoniae(top) and Pseudomonas aeruginosa (bottom). Data are from Scheld [18].
Conclusions
Several new developments in the pathogenesis and treatment of AECOPD now allow us to make judicious, evidence-based decisions with regard to the use of fluoroquinolones in the treatment of this common syndrome. Patients who are at risk for a poor outcome of the exacerbation and who are at risk of being infected with drug-resistant pathogens are good candidates for initial empirical treatment with a fluoroquinolone. For such patients, moxifloxacin is an appropriate choice because of the results of the MOSAIC trial, its excellent PK/PD properties, and its record of safety.
Acknowledgments
Financial support. This paper was generated from a symposium, “Moxifloxacin: An Assessment after 4 Years of Clinical Use” (16–18 April 2004; Naples, FL), through an unrestricted educational grant from Bayer Pharmaceuticals (West Haven, CT).
Potential conflicts of interest. S.S.: Honoraria for advisory boards of and/or speaking engagements from pharmaceutical manufacturers of antibiotics discussed in this manuscript.




![Life-table analysis of the time to the first composite event (treatment failure, and/or new exacerbation, and/or any further antibiotic treatment), stratified according to the time of the last acute exacerbation of chronic bronchitis (AECB) prior to randomization. Reproduced with permission from the American College of Chest Physicians [15].](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/cid/41/Supplement_2/10.1086_428058/1/m_41-Supplement_2-S177-fig002.gif?Expires=1716320621&Signature=42ADhpxGMF5cbKv9NeMg828bQTpeXgIVDIUlYktl7Z-YLghTJ9w2K1PfSSQvPHgq1LxrJqeMgjGjQIgXYQqUpdk6QcMI9O5V6V9S18pWCaksJc-R0KTHjua7MVW4m~8v7aRxgnVgZ36G1cOasjChSp3xREslqfnESzp9ESabF2~6QmGEfnfdiZkuPJvD3i1PyrJTuyLtVMT02bacXYQ4im3hmBm57szlHuz6S2dIMY1VptVys-ZzPEY9xcA9HOituUeBsFaJBNngKu46vEfuk5jaILEMbunbBjhbFQCsDbhV6bq42Bnob1Umv4c6R5X8ZJZ~Xh6PIYOFsgbwKnV3Jw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Area under the inhibitory curve (AUIC) for fluoroquinolones used to treat respiratory infections due to Streptococcus pneumoniae(top) and Pseudomonas aeruginosa (bottom). Data are from Scheld [18].](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/cid/41/Supplement_2/10.1086_428058/1/m_41-Supplement_2-S177-fig003.gif?Expires=1716320621&Signature=Vv~o5Gw9~naSk-biFvBKLcvISWrLqMNSg4qCbuwaSpEyhfhl~5bmf-30TXdrLIJN5bzTtvPVpIdsRiFKGrG1Y-T6nSzKlRdiLX9LLeQ~p9CXEQ1XmEzCmCY648SvYpKiKGTF2n8FOr0pd~U5vZAzabE1ioqhMuIoZ1ac~5zj3XpLRLTSLHD4yx65xy5hm4XrA558cgcIlIRJCDGmUGQmUIdSwG966LeKircWayOI~vqRA7w1SYlrAYP26oChYLgXmPdn-ge7T9-m9gSp3NLgJgaVesXu-Lf2ZV5yFGay8AhuIFPOq-xpS3jTJnL4AHMkFASxCwW6O~~qo76FYFOaYg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

Comments