Article Text
Abstract
Background: A study was undertaken to determine whether a short course of antibiotic treatment (⩽5 days) is as effective as the conventional longer treatment in acute exacerbations of chronic bronchitis and chronic obstructive pulmonary disease (COPD).
Methods: MEDLINE, EMBASE and the Cochrane central register of controlled trials were searched to July 2006. Studies considered eligible were double-blind randomised clinical trials including adult patients ⩾18 years of age with a clinical diagnosis of exacerbation of COPD or chronic bronchitis, no antimicrobial therapy at the time of diagnosis and random assignment to antibiotic treatment for ⩽5 days versus >5 days. The primary outcome measure was clinical cure at early follow-up on an intention-to-treat basis.
Results: 21 studies with a total of 10 698 patients were included. The average quality of the studies was high: the mean (SD) Jadad score was 3.9 (0.9). At early follow-up (<25 days), the summary odds ratio (OR) for clinical cure with short treatment versus conventional treatment was 0.99 (95% CI 0.90 to 1.08). At late follow-up the summary OR was 1.0 (95% CI 0.91 to 1.10) and the summary OR for bacteriological cure was 1.05 (95% CI 0.87 to 1.26). Similar summary ORs were observed for early cure in trials with the same antibiotic in both arms and in studies grouped by the antibiotic class used in the short-course arm.
Conclusions: A short course of antibiotic treatment is as effective as the traditional longer treatment in patients with mild to moderate exacerbations of chronic bronchitis and COPD.
Statistics from Altmetric.com
Chronic bronchitis is one of the five leading causes of death worldwide and affects 3–17% of the adult population in developed countries.1 Acute exacerbations of chronic bronchitis and chronic obstructive pulmonary disease (COPD) occur frequently. Causes include air pollutants, allergens and viruses, as well as bacterial pathogens. The predominant bacterial pathogen implicated in acute exacerbations of chronic bronchitis and COPD is Haemophilus influenzae, which is present in 50% of all bacterial exacerbations, with approximately a further one-third of isolates being either Streptococcus pneumoniae or Moraxella catarrhalis.2
Most patients with acute exacerbations of chronic bronchitis and COPD are treated with antibiotics, but the benefit of antibiotic therapy remains controversial. This controversy is fuelled by data suggesting that at least one-third of exacerbations are non-infectious in origin.3–5 In addition, clinical trials of antibiotics have yielded conflicting data, with several large studies failing to demonstrate superiority of antibiotic therapy over placebo.6 7 Other trials indicated that antibiotic therapy is effective in patients who have at least two of the following symptoms: increased dyspnoea, increased sputum volume and increased sputum purulence (ie, a type 1 or 2 exacerbation),8 and in those with more severe airflow obstruction.9 A meta-analysis recently included in the Cochrane Library confirms these findings by demonstrating that, in acute exacerbations of chronic bronchitis and COPD with increased cough and sputum purulence, the use of antibiotics reduces the risk of short-term mortality by 77% and decreases the risk of treatment failure by 53%.10
This raises the question of how long the duration of antibiotic therapy should be. Antibiotic consumption in acute exacerbations of chronic bronchitis and COPD occurs on large scale and this may contribute to increasing resistance rates of the relevant pathogens.11 12 Up to 27% of H influenzae and >90% of M catarrhalis produce β-lactamases, with large geographical differences.13 14 Worldwide, the overall prevalence of penicillin non-susceptibility among strains of S pneumoniae is around 30–40%, with geographical variations between 2% and 60%.15–17 The geographical differences can at least in part be attributed to differences in overall antibiotic consumption.12 A shorter duration of treatment might help contain these growing resistance rates, but shorter treatment can only be recommended if this is equally efficacious.
We performed a systematic review and meta-analysis of published randomised double-blind studies to determine whether a short course of antibiotic treatment is as effective as a long course in patients with a type 1 or 2 exacerbation of COPD/chronic bronchitis.
METHODS
Criteria for considering studies for this review and primary outcomes
Studies considered eligible for inclusion were randomised trials of antibiotic intervention involving adult patients ⩾18 years of age with a diagnosis of COPD or chronic bronchitis. Studies not published in the English language were excluded.
The primary outcome was clinical cure at early follow-up (as defined by the authors of the studies), defined as resolution or improvement of the clinical symptoms of the exacerbation. Treatment failure included lack of clinical resolution or improvement and indeterminate outcome: clinical response to the study drug could not be assessed for any reason. Secondary outcomes were: (1) the rate of clinical cure reported from the time of diagnosis to the final evaluation point (late follow-up): treatment failures included recurrences, relapses and indeterminate cases; and (2) the bacteriological cure rate. Bacteriological failure included persistence of the causative pathogen, presumed persistence (if no material was available for culture in a patient considered a clinical failure), and indeterminate outcome (if the bacteriological response to the study drug was not evaluable for any reason).
Search strategy for identification of studies
We searched the Cochrane central register of controlled trials on the Cochrane Library (Issue 2, 2006), Medline (1966–July 2006) and Embase (1988–July 2006) using the following search terms: chronic bronchitis or COPD, antibiotic treatment and clinical trials (see appendix A for details of the search strategy). We also searched the reference lists of included studies for additional studies.
Data extraction
Studies were included in the meta-analysis if they satisfied the following criteria: (1) adult patients ⩾18 years of age; (2) clinical diagnosis of exacerbation of COPD, chronic bronchitis or pulmonary emphysema; (3) no antimicrobial therapy at the time of diagnosis; (4) random assignment to antibiotic treatment for ⩽5 days versus treatment for >5 days; (5) study design with double blinding. Double-blind studies with azithromycin in the short arm were excluded. This antibiotic has a very long half-life and 3 days of treatment with azithromycin can therefore not be regarded as a short therapy.
Two authors independently rated abstracts identified by the electronic searches for inclusion in the meta-analysis. Inter-rater reliability for trial selection was assessed with Cohen’s κ. In cases of disagreement between raters, the full original article was retrieved for data extraction. Differences in opinion over inclusion of studies were resolved through discussions and consensus.
Hard copies of the full article of all potentially eligible studies were obtained. Two reviewers independently extracted the following data from each study: author, year of publication, sample size, mean age of subjects, percentage of smokers, hospitalised or outpatient status of the subjects, antibiotic regimen used, antibiotic treatment duration, criteria used to define exacerbation of chronic bronchitis or COPD and the major outcome measure(s) for each study.
Assessment of study quality
The internal validity of included trials was assessed by the same reviewers using the Jadad scale.18 The scale assigns scores from 0 to 5 (best quality trial) based on the following items: (1) the study is randomised; (2) the intervention is double blind; (3) an accounting and description of study withdrawals is done; (4) the randomisation procedure is adequately performed using an appropriate method such as computer generated random numbers; and (5) the blindness is also adequately performed using identical-looking placebo.
Concealment of treatment allocation was also evaluated for adequacy: if trialists were unaware of each participant’s allocation when they were recruited, the allocation was said to be adequately concealed.
Statistical analysis
Meta-analyses were performed with the Cochrane collaboration’s Revman 4.1 program (Cochrane Collaboration, Oxford, UK). From each study the clinical and bacterial cure rates were calculated and the chance of cure with a short course of antibiotics (⩽5 days) compared with a longer course (>5 days) was expressed as an odds ratio (OR) with 95% confidence intervals (CIs). An OR of <1 indicates a lower number of cured cases with the short course of antibiotics and superiority of the long course of antibiotics.
Summary ORs were calculated based on the individual trial outcomes using the fixed-effect model. In additional analyses, studies were grouped by the class of antibiotic used in the short-course arm: cephalosporins, macrolides (other than azithromycin) and fluoroquinolones. Statistical heterogeneity among trials was assessed by χ2 analysis. The presence of publication bias was assessed by a funnel plot.
Sensitivity analyses were conducted to assess the robustness of the study by comparing summary ORs among groups redefined by (1) excluding trials of a lower methodological quality (Jadad score <4); (2) excluding trials with inadequate or unknown concealment; and (3) excluding trials of comparisons between different antibiotics.
RESULTS
Literature search and trial inclusion
The search strategy identified 885 studies. A total of 30 full hard copies were selected for further data extraction (fig 1). There was 94% agreement about which abstracts to include for retrieval of hard copies (κ = 0.79, 95% CI 0.66 to 0.92).
Of these potentially eligible studies, 21 met the criteria for inclusion in the meta-analysis. Details of studies comparing short antibiotic treatment with conventional treatment (7–10 days) in patients with acute exacerbations of chronic bronchitis and COPD are shown in table 1.
Three trials had three treatment arms.19–21 In these cases the comparison of the short and long duration with the same antibiotic was chosen above the comparison between different antibiotics. Two trials were reported in a single paper.22 As sufficient information could be extracted from this paper, they were included in the meta-analysis.
Methodological quality
The mean (SD) quality score for the trials was 3.9 (0.9) on the Jadad scale; 71% were of very high quality (Jadad score ⩾4; table 1). Substantial inter-rater agreement for assignment of this score was reached (κ = 0.75, 95% CI 0.60 to 0.90). Seventeen studies (81%) described the reasons for patient withdrawal. Six trials (29%) were judged as having adequate allocation concealment (table 1); the remaining studies did not describe the concealment of treatment allocation.
Description of trials
The 21 included studies included a total of 10 698 patients (table 1), of which 5348 patients were allocated to short treatment groups and 5350 to long treatment groups. Four trials did not specify how exacerbation was defined.20–22 All other trials included only patients satisfying at least two of the following criteria: increased cough and/or dyspnoea, increased sputum volume and increased purulence (ie, a type 1 or 2 exacerbation as defined by Anthonisen et al8). The mean (SD) age of study patients was 57.4 (4.3) years in the short treatment groups and 58 (4.4) years in the long treatment groups. The mean (SD) percentage of smokers in the short treatment and long treatment groups was 71.8 (16.3)% and 71.8 (16.3)%, respectively. Most of the studies included outpatients. The mean (SD) duration of treatment was 4.9 (0.4) days in the short treatment groups and 8.3 (1.5) days in the long treatment groups (table 1).
All trials defined clinical cure as the disappearance of acute exacerbations of chronic bronchitis and COPD-related signs and symptoms, return to the pre-infection state, or sufficient improvement such that additional or alternative antimicrobial therapy was not required. The mean (SD) time of early follow-up evaluation was after 15 (3.5) days and the mean (SD) time of late evaluation was at day 31 (5.3). In all but one trial clinical cure rates at early follow-up could be extracted or calculated.20 Four studies did not report clinical cure rates at late follow-up.20 23–25 Three studies did not report bacteriological cure rates.23 26 27
Outcome of clinical and bacteriological cure rates
The primary outcome analysed was the clinical cure rate at early follow-up in an intention-to-treat (ITT) analysis. Early follow-up was before day 25 in all studies. Tests for statistical heterogeneity were performed for all analyses. Statistically significant heterogeneity was not observed in the primary outcome of early clinical cure (p = 0.8) or in the secondary outcomes of late clinical cure (p = 0.34) and bacteriological cure (p = 0.2). A funnel plot did not suggest any form of publication bias.
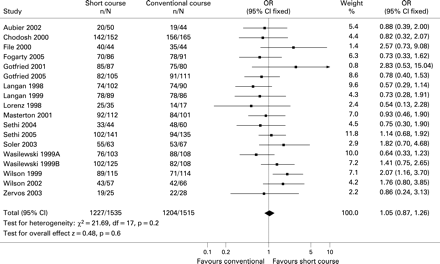
The summary OR for early treatment cure was 0.99 (95% CI 0.90 to 1.08) (fig 2). In trials with the same antibiotic in both arms, the summary OR was 0.93 (95% CI 0.78 to 1.11) (fig 3). The summary OR for cured cases at late follow-up was 1.0 (95% CI 0.91 to 1.10) and for bacteriological cure was 1.05 (95% CI 0.87 to 1.26) (fig 4).
Similar summary ORs were observed for early clinical cure in studies grouped by the antibiotic class used in the short arm. The summary OR was 1.04 (95% CI 0.87 to 1.24) for cephalosporins, 0.96 (95% CI 0.80 to 1.16) for macrolides and 0.94 (95% CI 0.81 to 1.09) for fluoroquinolones (fig 5).
Sensitivity analysis
Sensitivity analyses were conducted for the primary outcome (ie, clinical cure rate at the early follow-up). Treatment cure rates were not significantly more likely with shorter duration in very high quality trials (summary OR 1.02, 95% CI 0.91 to 1.13), trials with adequate concealment (summary OR 1.04, 95% CI 0.87 to 1.24) or in trials with the same antibiotic in both arms (summary OR 0.93, 95% CI 0.78 to 1.11).
DISCUSSION
In this systematic review of randomised double-blind studies we found that short courses of antibiotic therapy (⩽5 days) are as effective as the conventional courses of >5 days in the treatment of mild to moderate acute exacerbations of chronic bronchitis and COPD. The clinical cure rates at both early and late follow-up as well as the bacteriological cure rates were comparable for both treatment strategies. Similar summary ORs were found for early cure in trials with the same antibiotic in both arms.
The overall methodological quality of studies in our systematic review was found to be high or very high, with 71% of the studies having a Jadad score of at least 4. Sensitivity analyses showed no differences between both treatment groups, enhancing the statistical robustness of the overall analysis and strengthening the appropriateness of combining all studies into a single meta-analysis. Statistically significant heterogeneity was not present.
A potential weakness of meta-analyses is the incorporation of existing biases and the introduction of new biases.28–30 To minimise bias during trial selection we used predetermined inclusion criteria. Language bias must be considered since this meta-analysis included only trials published in the English language. No signs of publication bias were detected.
Different antibiotic classes are represented, but new agents were often used in the short treatment arm. Studying amoxicillin-clavulanic acid (co-amoxiclav) and tetracycline/doxycycline in the short arm would be relevant. However, on microbiological or pharmacological grounds, there are no reasons to believe the results are different for these agents. Most of the studies included in the meta-analysis were conducted in the community, although at least four studies also included hospital inpatients. Although almost all exacerbations were classified as Anthonisen type 1 or 2, we feel some caution is necessary when applying our findings to patients with severe exacerbations who are admitted to hospital with respiratory failure.
Antibiotics are widely prescribed for respiratory tract infections, which account for 75% of community prescriptions.31 32 Tonsillopharyngitis is the most frequent indication, followed by bronchitis. It has already been demonstrated that a short course (4–5 days) of treatment with a cephalosporin is at least as effective as 10 days of penicillin treatment in patients with group A streptococcal tonsillopharyngitis,33 and that a short course (5 days) of short-acting antibiotics is an effective treatment for uncomplicated acute otitis media in children.34 Studies investigating the effectiveness of shorter courses in community-acquired pneumonia show promising results.35–38
According to most guidelines on the treatment with COPD, antibiotic treatment is only indicated in patients with acute exacerbations of COPD characterised by increased sputum volume and purulence.3–5 39 The issue of the appropriate duration of antibiotic therapy, however, is not addressed in any of these guidelines.
Shorter courses of antibiotic treatment have several potential advantages compared with long courses of treatment. Poor compliance appears to be more common with longer treatment courses, so shorter courses of antibiotics may enhance compliance. The compliance rate in tonsillopharyngitis, in which penicillin therapy is typically prescribed for 10 days, is inversely related to the duration of treatment and has been observed to be as low as 8% by the ninth day of treatment.40–42 It is to be expected that a short course of treatment will also reduce antibiotic costs.
More important is the effect of unnecessarily lengthy courses on the development of resistant organisms. On a population level, there is a clear relationship between total antibiotic consumption and resistance rates of pathogens.11 12 43 44 Decreasing the duration of antibiotic courses in respiratory tract infections might contribute to a decrease in these resistance rates.44
Our meta-analysis supports the effectiveness of short-course treatment in mild to moderate exacerbations of COPD or chronic bronchitis characterised by at least two of the following criteria: increased cough and/or dyspnoea, increased sputum volume and increased purulence. Based on the included studies, it seems that the duration of antibiotic treatment can be safely reduced. We therefore propose that the guidelines for COPD should recommend antibiotic treatment duration of no longer than 5 days, regardless of antibiotic class, in mild to moderate exacerbations of COPD or chronic bronchitis.
Acknowledgments
The authors thank Heleen C Dyserinck for her support with the search strategy.
Appendix A
Electronic search strategy using Medline:
((“Pulmonary Disease, Chronic Obstructive”[MeSH]) OR (chronic bronchitis) OR (pulmonary emphysema) OR (chronic obstructive bronchitis*) OR (chronic airflow limitation*) OR (chronic airflow obstruction*) OR (obstructive airways disease*) OR (chronic obstructive lung disease*))
AND
((“Anti-Bacterial Agents”[MeSH] OR “Anti-Bacterial Agents”[Pharmacological Action]) OR (antibiotic*))
AND
((randomized controlled trial[pt] OR controlled clinical trial[pt] OR randomized controlled trials[mh] OR random allocation[mh] OR double-blind method[mh] OR single-blind method[mh] OR clinical trial[pt] OR clinical trials[mh] OR “clinical trial”[tw] OR ((singl*[tw] OR doubl*[tw] OR trebl*[tw] OR tripl*[tw])
AND
(mask*[tw] OR blind*[tw])) OR “latin square”[tw] OR placebos[mh] OR placebo*[tw] OR random*[tw] OR research design[mh:noexp] OR comparative study[mh] OR evaluation studies[mh] OR follow-up studies[mh] OR prospective studies[mh] OR cross-over studies[mh] OR control*[tw] OR prospectiv*[tw] OR volunteer*[tw]) NOT (animal[mh] NOT human[mh])) Limits: English
Electronic search strategy using Ovid
1. exp Chronic Obstructive Lung Disease/
2. exp Chronic Bronchitis/
3. exp lung emphysema/
4. chronic bronchitis.tw.
5. lung emphysema.tw.
6. chronic obstructive lung disease.tw.
7. pulmonary emphysema.tw.
8. 1 or 2 or 3 or 4 or 5 or 6 or 7
9. exp Antibiotic Agent/
10. 8 and 9
11. random$.tw.
12. 10 and 11
13. limit 12 to (human and English language)
REFERENCES
Footnotes
The sponsors of the study had no role in study design, data collection, data analysis, data interpretation or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Funding: None.
Competing interests: Healthcare Insurance Board, Amstelveen, The Netherlands (grant OG99-038).